Chapter 8 Practice Problems – Answers
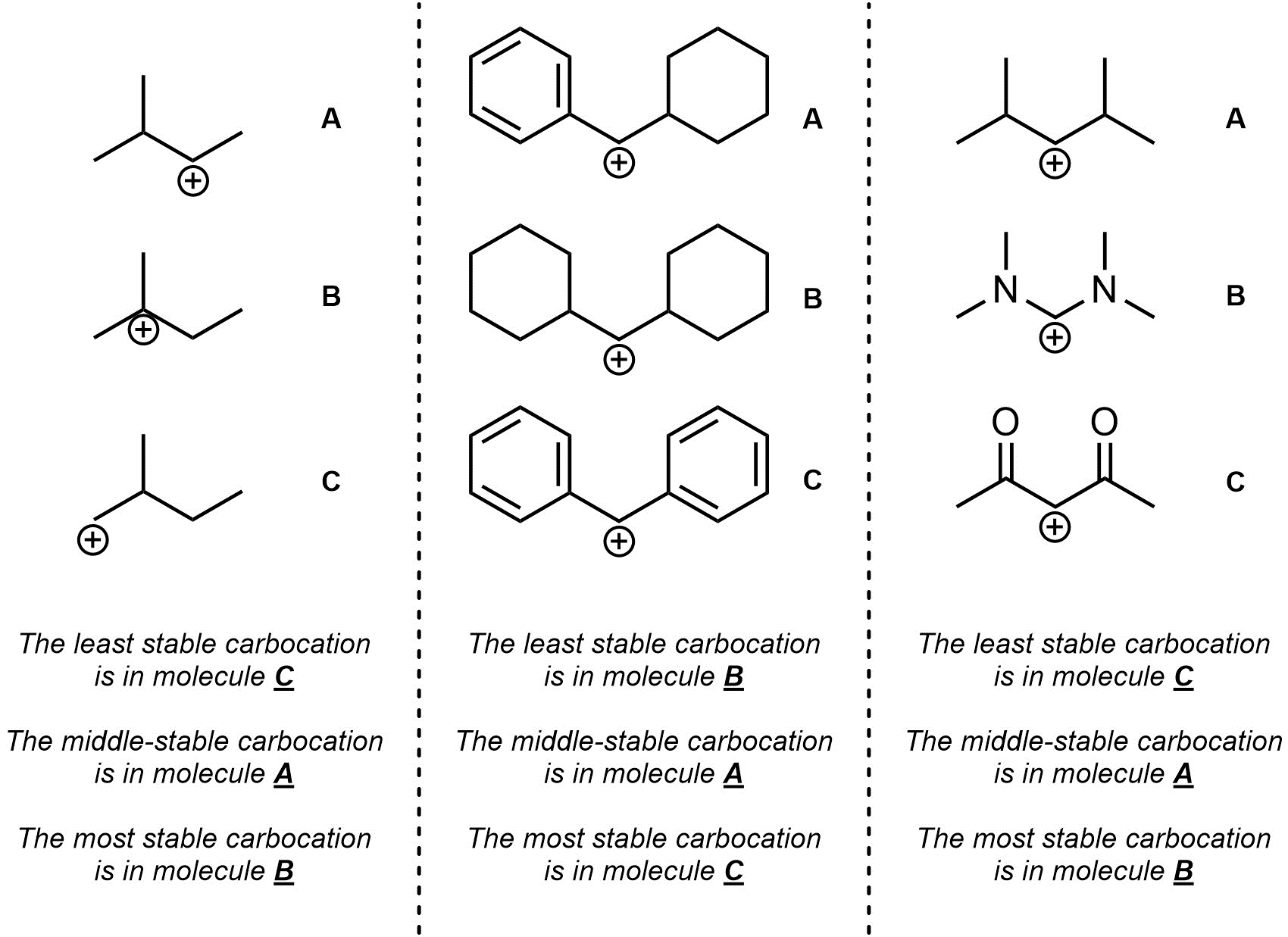
Q8.1: Rank each set of carbocations in increasing order of stability (least to most stable).
For the third question think critically about resonance and induction.
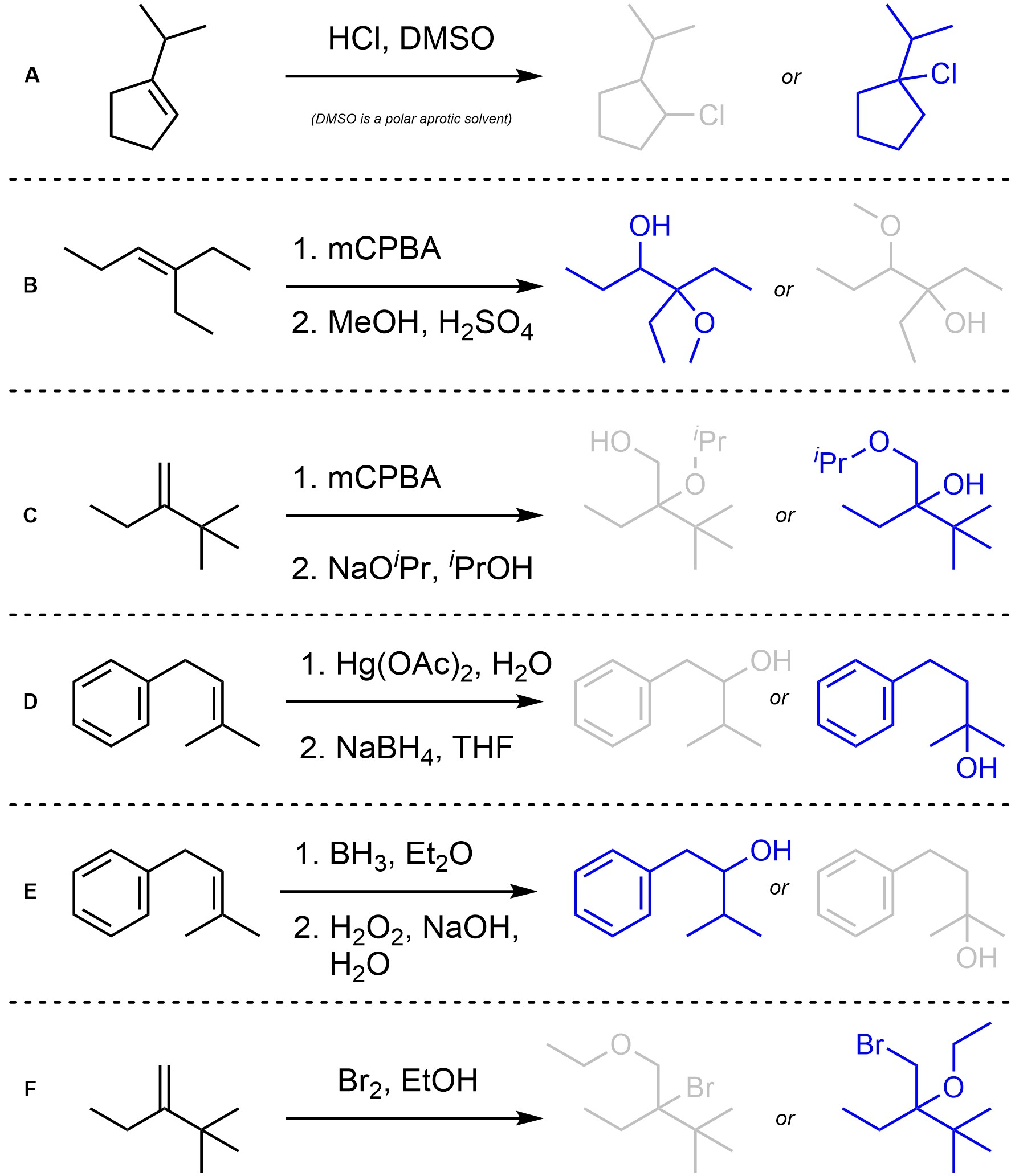
Q8.2: Assume each of the following reactions proceeds as expected. For each reaction indicate (circle, draw an arrow to, etc) which regioisomer will be the major product. You may ignore stereochemistry for this question.
Consider Markovnikov vs. Anti-Markovnikov selectivity.
Major regioisomer highlighted in blue.
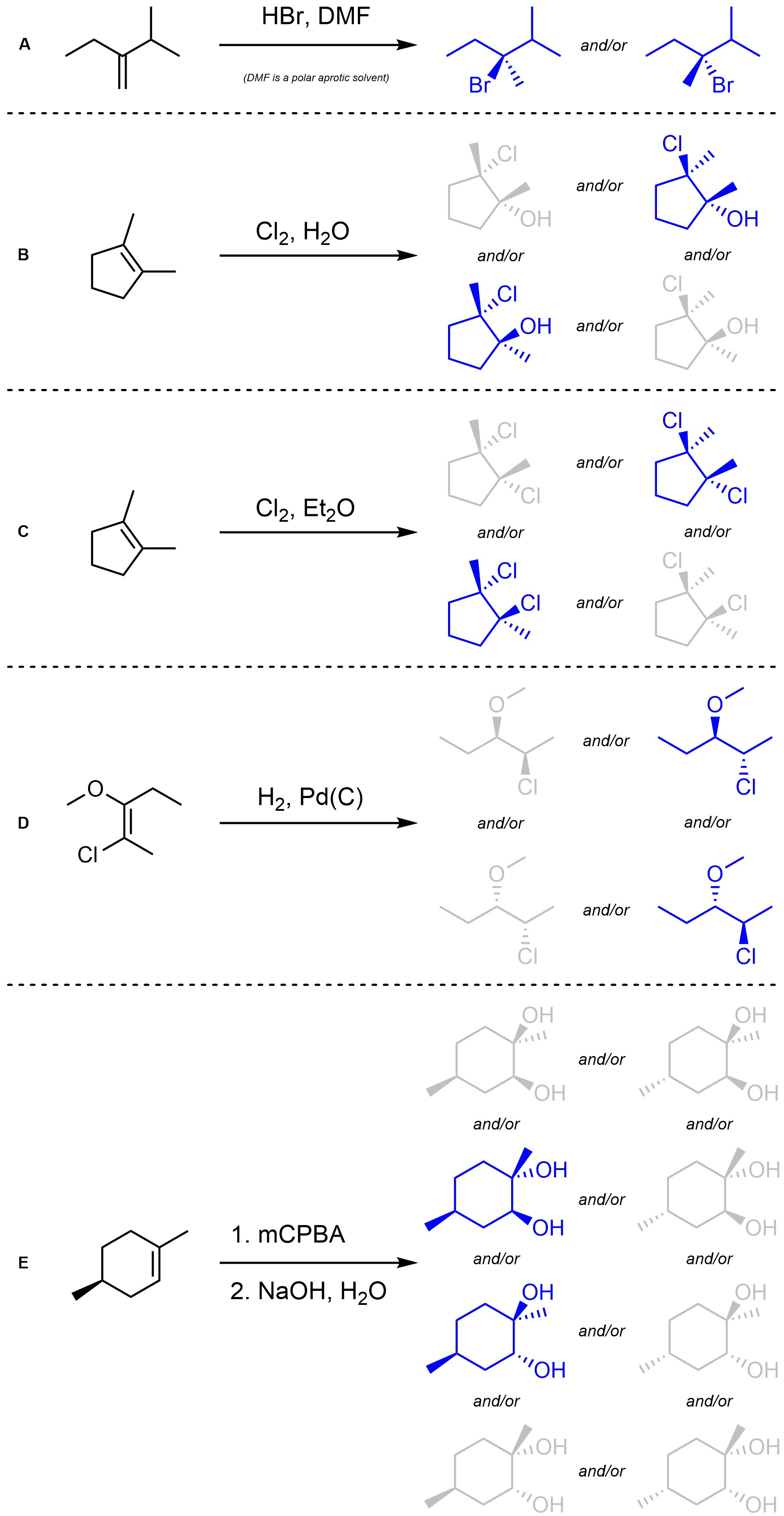
Q8.3: Assume each of the following reactions proceeds as expected. For each reaction indicate (circle, draw an arrow to, etc) which stereoisomer(s) will be the major product(s). You may ignore regioselectivity for this question.
Remember conformation can change but absolute configurations do not.
Consider whether pre-existing stereocentres will be changed.
Major stereoisomer(s) highlighted in blue.
Q8.4: For each reaction draw the expected major organic product(s). The products must be drawn as line-angle structures. Clearly indicate the appropriate stereochemistry using hashed and wedged bonds where necessary. If the product(s) is/are formed as more than one diastereomer, draw all diastereomers formed. If the product(s) is/are formed as a racemate (racemic mixture) draw only one enantiomer and write the word “racemic” beneath/beside it. Do not write “racemic” if it does not apply.
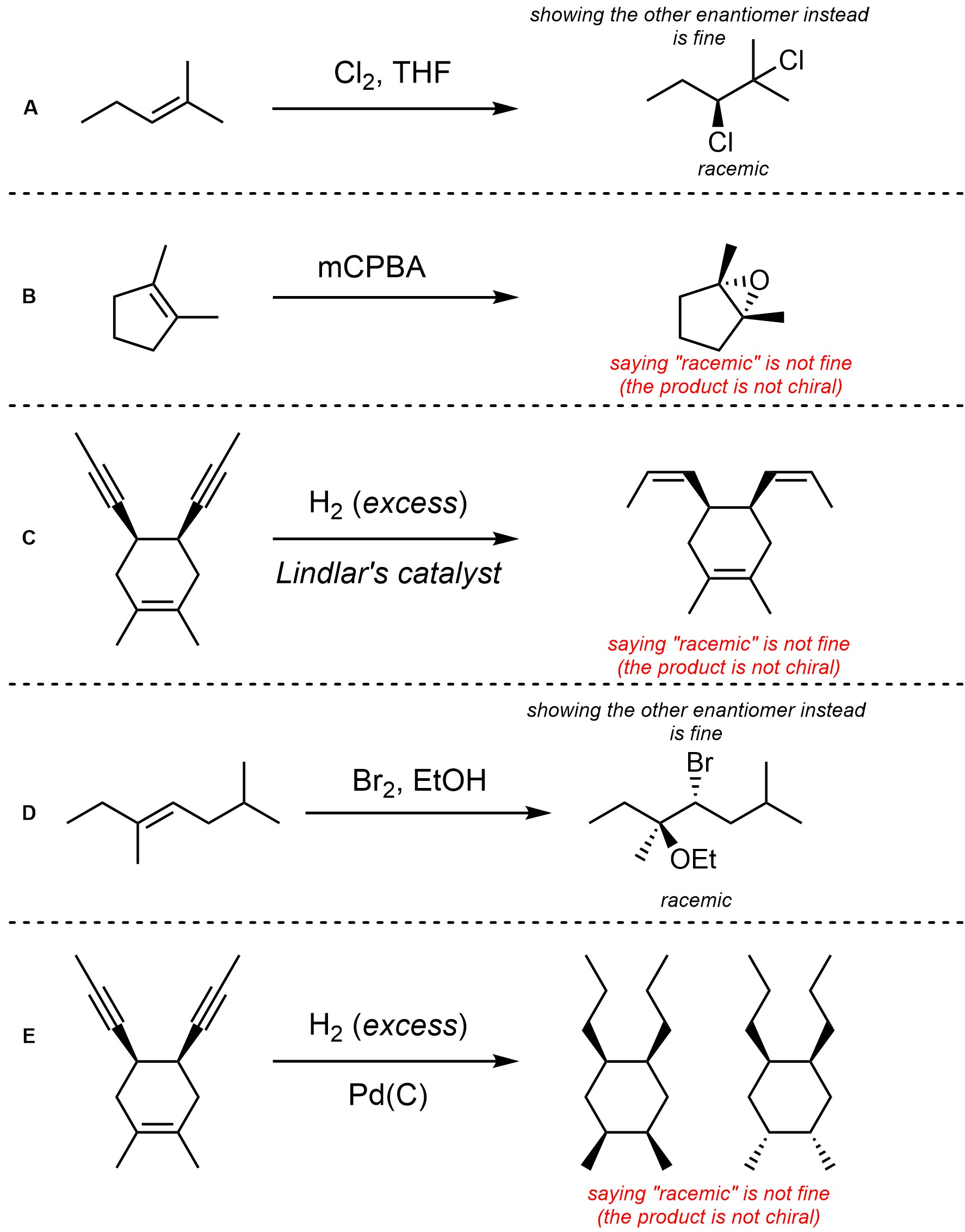
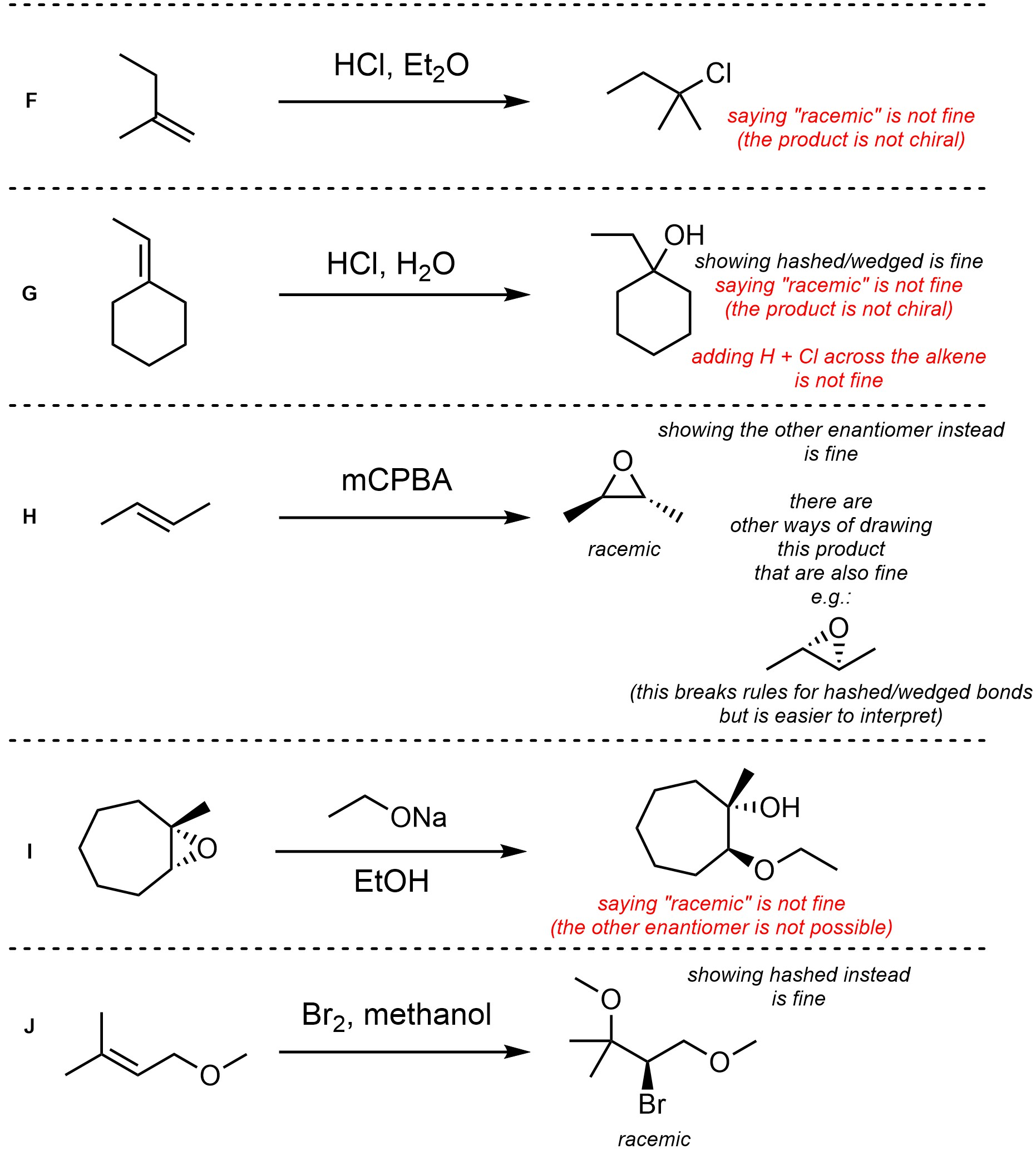
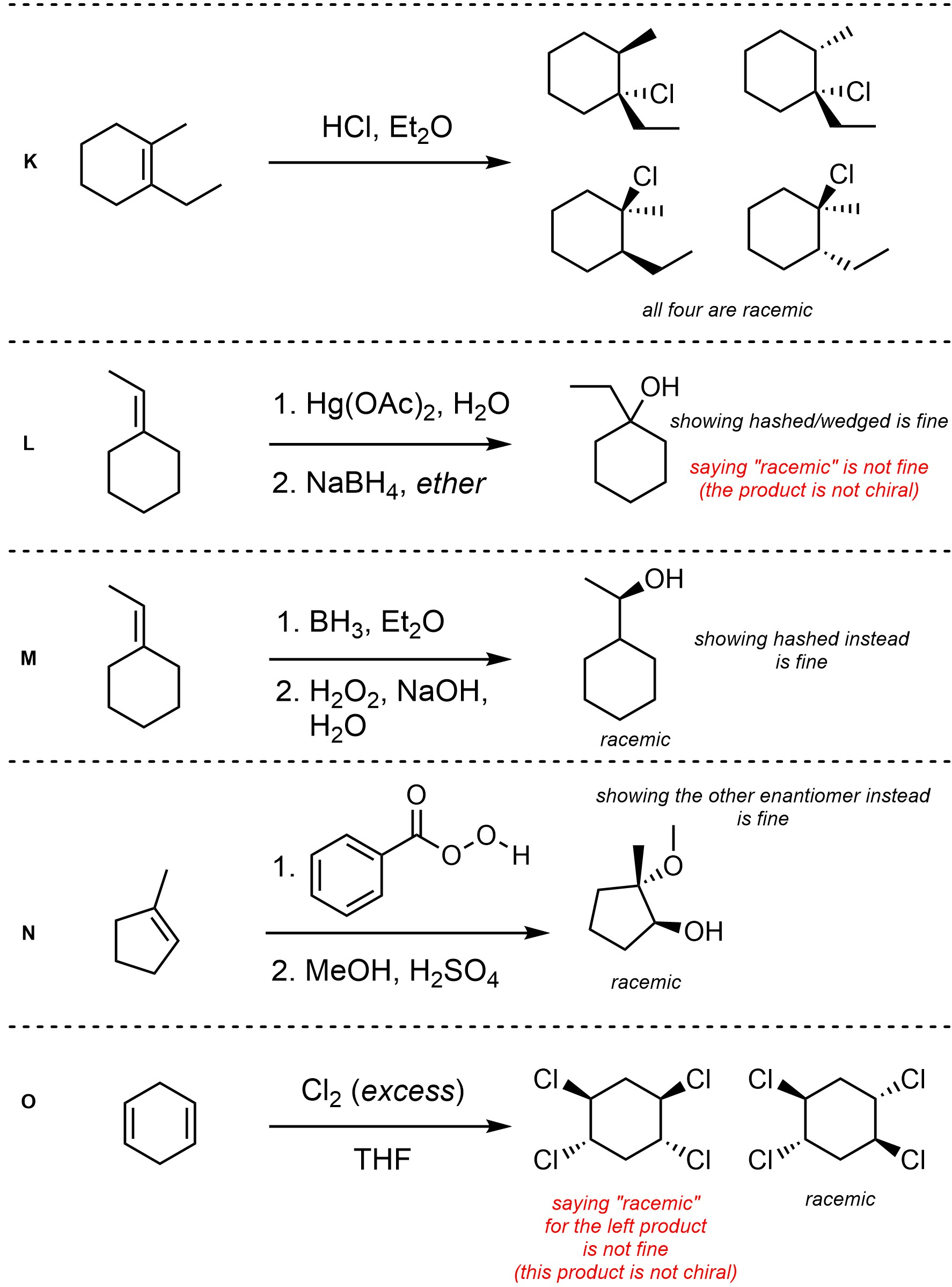
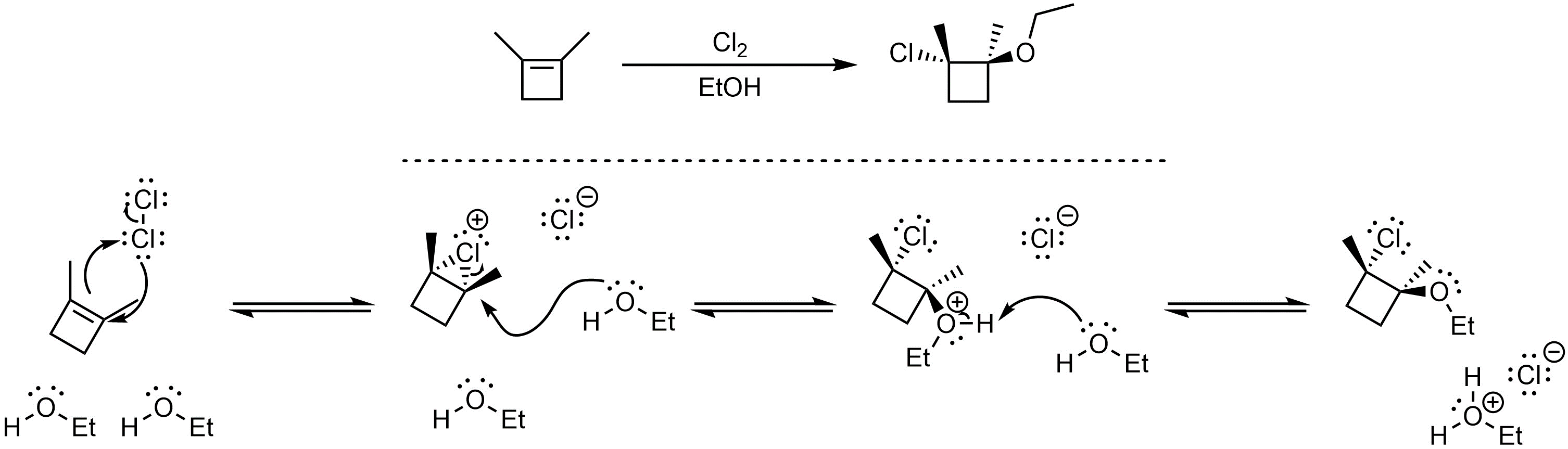
Q8.5: A reaction equation is given below. Propose a reasonable mechanism for the reaction. Show all necessary intermediates, curved arrows, lone pairs, and formal charges. If there is a catalyst remember to regenerate it at the end of the reaction.
Mechanism is the same as Scheme 8.26 in Section 8.8.1