12.7. Oxidation of Alcohols via Elimination
Recall that in organic chemistry oxidation and reduction are commonly associated with the loss or gain of hydrogens (see Section 7.4). For example, the conversion of a carbonyl to an alcohol is considered a reduction reaction, while the reverse would be considered an oxidation reaction.
Some oxidation reactions, typically those of alcohols and hydrates, are equivalent to elimination reactions (Scheme 12.15).
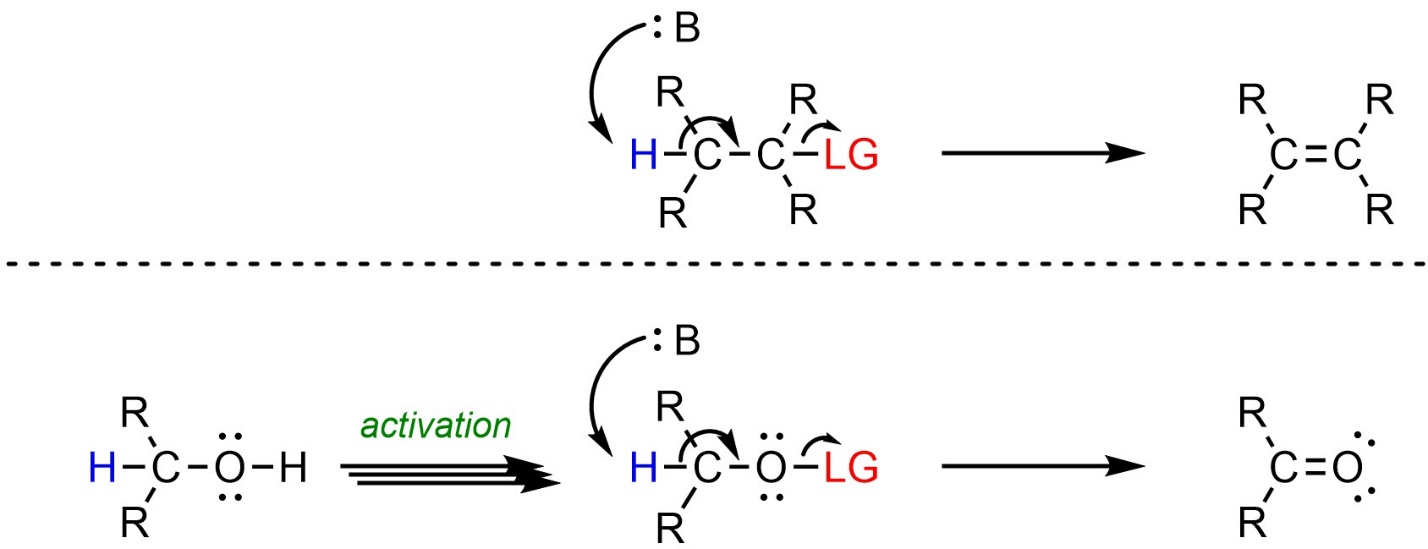
Scheme 12.15 – Comparison of Generalized Reaction Mechanisms for Elimination and Some Oxidation Reactions.
12.7.1. Reaction: Oxidation using X-X and a Base
It is possible to oxidize an alcohol to an aldehyde or ketone using a dihalide (X2) and a weak base. The most common pairing is bromine (Br2) and sodium bicarbonate (NaCO3H). After activation this reaction follows an E2 mechanism (Scheme 12.16). First, the alcohol (nucleophile) attacks the dihalide (electrophile). This creates a new O-X bond and a salt. Second, the base removes a proton, creating the activated intermediate. Finally, another equivalent of base removes a proton to cause the elimination/oxidation step.
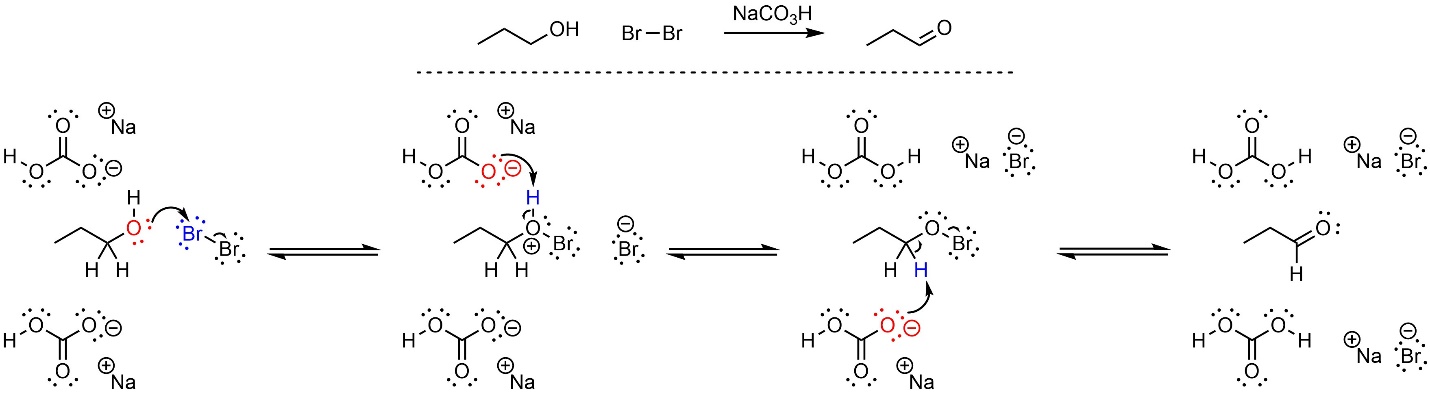
Scheme 12.16 – Reaction Mechanism for Oxidation of Propan-1-ol Using Bromine and Sodium Bicarbonate.
This oxidation works well for simple molecules but is not commonly used in complex syntheses; because a dihalide, water, and a base are present other simple reactions, such as additions across alkenes, can also occur.
12.7.2. Reaction: Oxidation using CrO3 (Jones Oxidation)
It is possible to oxidize an alcohol to a carboxylic acid or ketone using an acid and chromium trioxide (CrO3). This is sometimes referred to as Jones Oxidation. The actual chromium compound needed for the reaction (HCrO3+) can be generated several ways (Scheme 12.17); mixing chromium trioxide (CrO3), chromic acid (H2CrO4), or sodium dichromate (Na2Cr2O7) with sulfuric acid (H2SO4) will form the required intermediate. The mechanism for the reaction with sodium dichromate is straightforward but time consuming; at an introductory level it is important only to know that this reaction is possible with this chromium source. Some texts show the sulfuric acid directly reacting with the chromium trioxide, chromic acid, or sodium dichromate to form the protonated chromium trioxide. Since water is present, this is unlikely to be accurate.
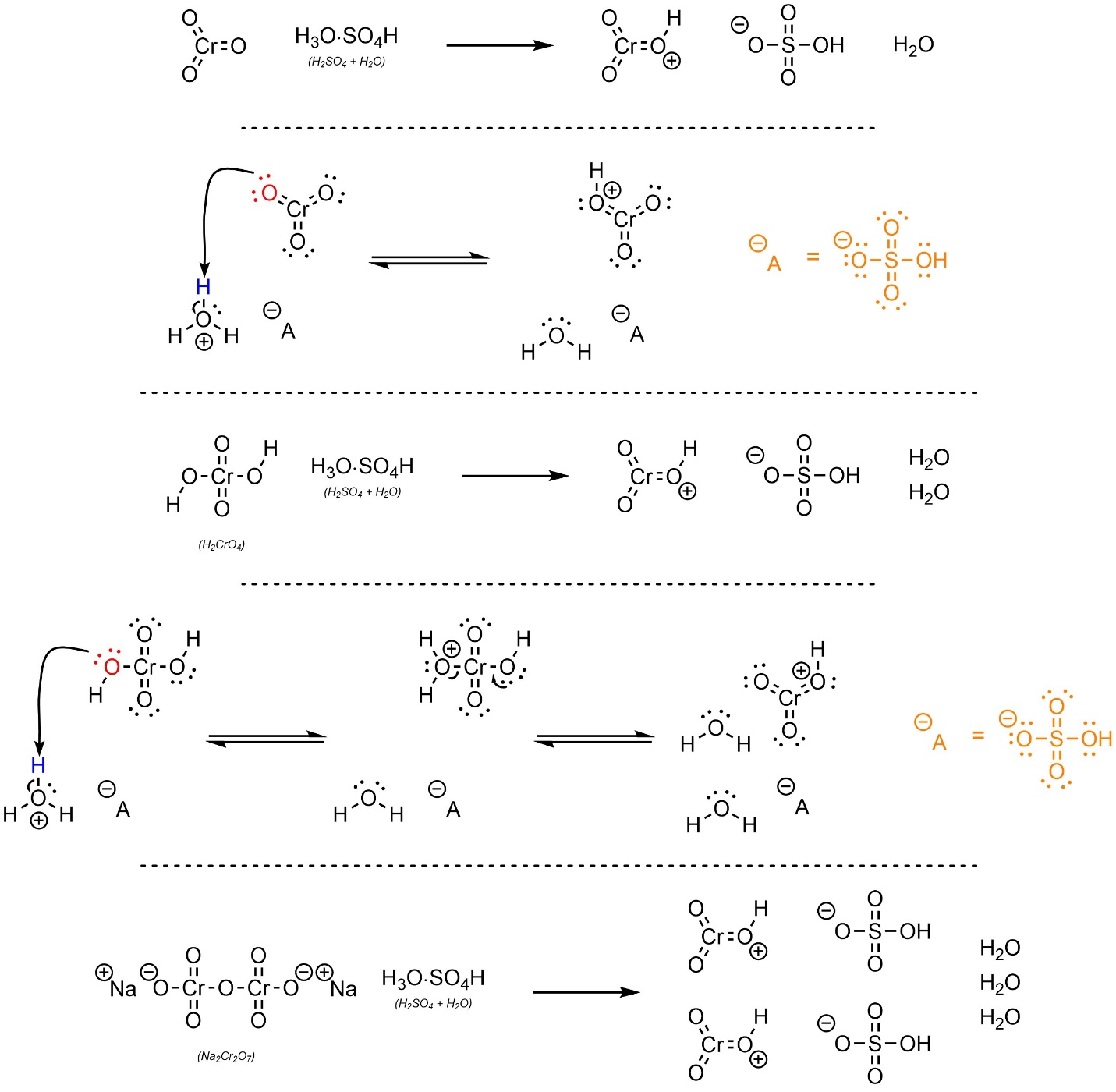
Scheme 12.17 – Formation of Activated Chromium Trioxide Using Sulfuric Acid and Chromium Trioxide, Chromic Acid, or Sodium Dichromate.
12.7.2.1. Mechanism
After formation of the activated chromium trioxide (not shown) this reaction follows an E2 mechanism (Scheme 12.18). First, the alcohol (nucleophile) attacks the activated chromium trioxide (HCrO3+; electrophile). This creates a new O-Cr bond and a salt. Second, the base (water) removes a proton, creating the activated intermediate. Finally, another equivalent of water removes a proton to cause the elimination/oxidation step.
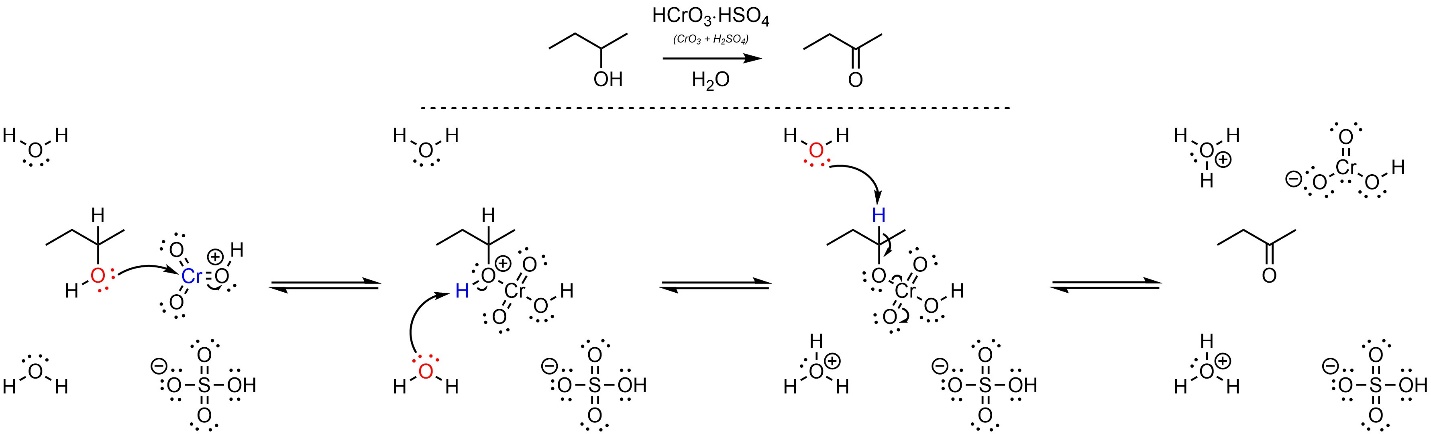
Scheme 12.18 – Reaction Mechanism for Oxidation of Butan-2-ol Using Protonated Chromium Trioxide in Water.
12.7.2.2. The Role of Water
It is important to remember that sulfuric acid (H2SO4) is always contaminated with at least a small amount of water (H2O). When water, acid, and aldehydes/ketones are present they will combine to form hydrates (see Section 7.5.2). With ketones this is not a problem, however with aldehydes the hydrate can undergo another oxidation (Scheme 12.19). As a result, any Jones oxidation reaction that has water in it will oxidize primary alcohols twice, into carboxylic acids, instead of forming the aldehyde. The mechanism for the second oxidation is identical to that of the first; drawing the complete mechanism for a Jones oxidation of a primary alcohol to a carboxylic acid is more tedious than challenging.
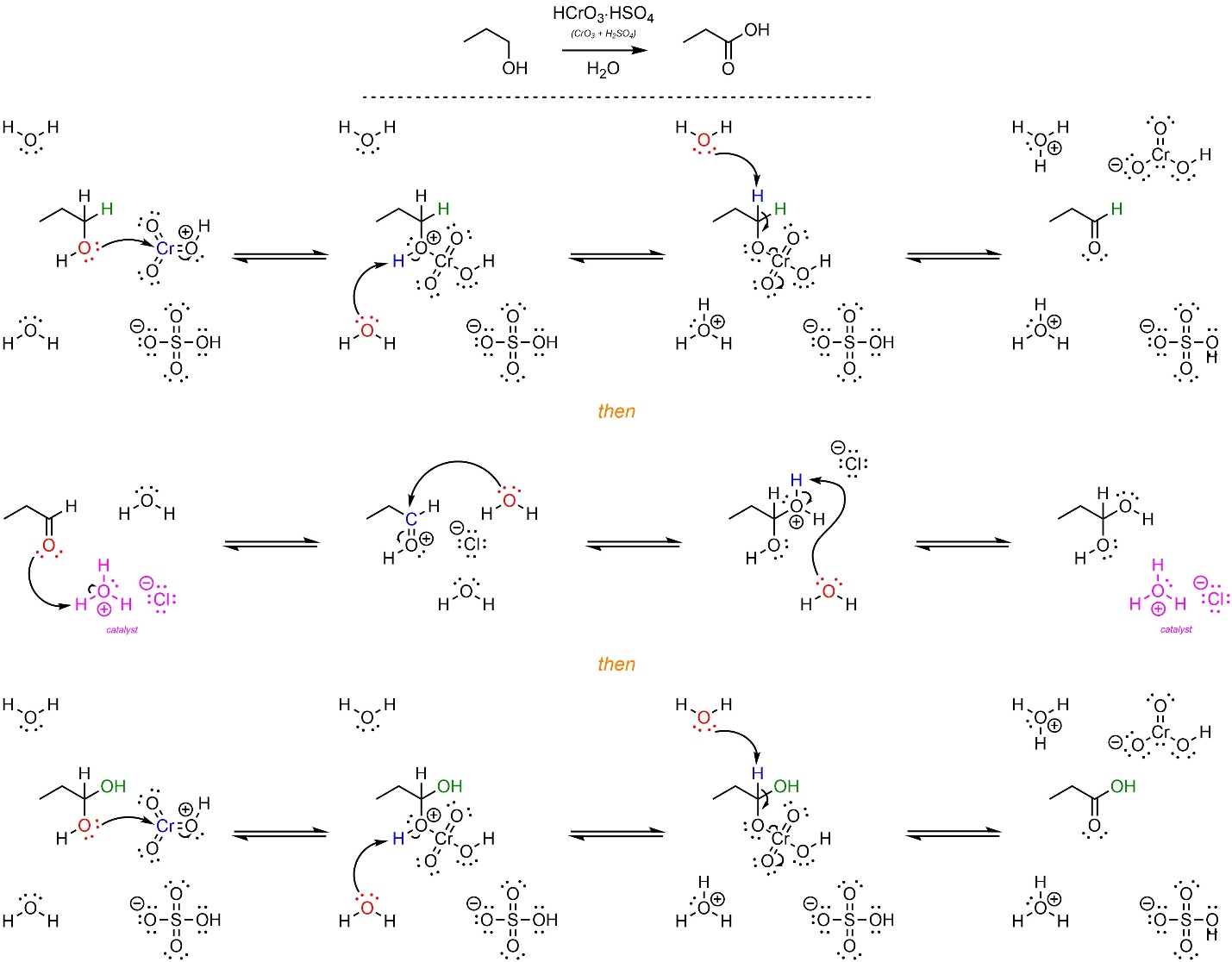
Scheme 12.19 – Reaction Mechanism for Oxidation of Propan-1-ol Using Protonated Chromium Trioxide in Water.
12.7.2.3. Solving Problems Using Special Reagents – PCC
It is possible to stop the oxidation of primary alcohols as aldehydes by rigorously excluding water. This can be accomplished by changing the way the activated chromium trioxide (HCrO3+) is formed. The most common approach is to use pyridinium chlorochromate (PCC) instead of combining a chromium source with sulfuric acid (Scheme 12.20).
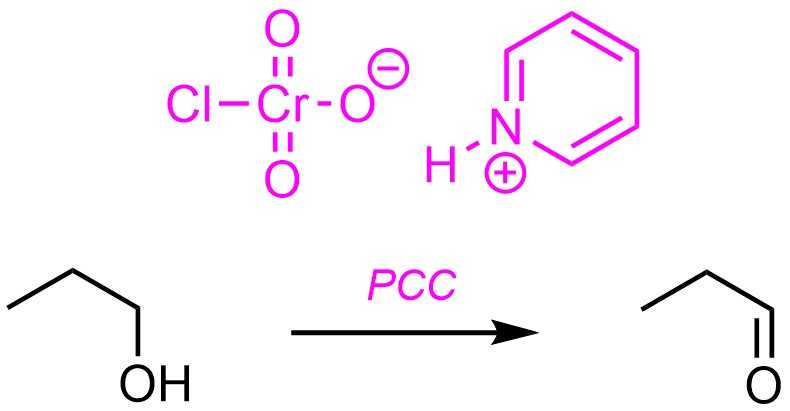
Scheme 12.20 – Reaction Equation for Oxidation of Propan-1-ol Using Pyridinium Chlorochromate (PCC).
PCC is stable while a solid. When dissolved in a solvent PCC spontaneously forms activated chromium trioxide in equilibrium with itself (Scheme 12.21). Because PCC does not include water, the hydrate cannot form and further oxidation cannot occur. The mechanism for the oxidation is identical to other Jones oxidation reactions.
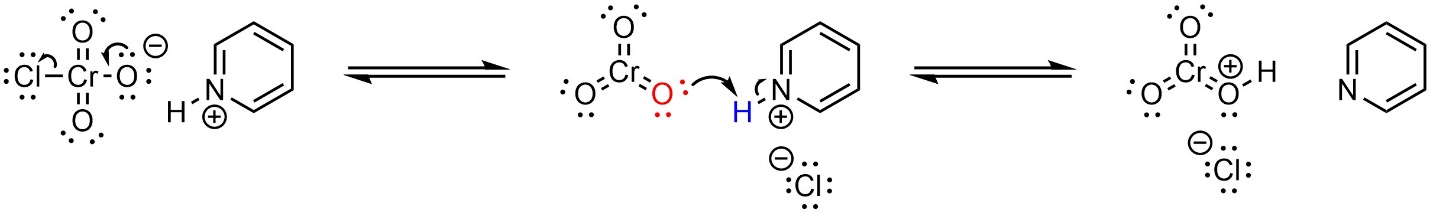
Scheme 12.21 – Mechanism for Formation of HCrO3+ from Pyridinium Chlorochromate (PCC).

