13.7. Reactions with Carboxylic Acid/Ester Electrophiles
Carboxylic acids and esters are similar in terms of electrophilicity. They are also very common in natural and synthetic compounds. As a result, a large amount of effort has gone into developing many reactions using these functional groups. This section includes more reactions and detail to emphasize their importance.
The mechanisms for several of these reactions are among the longest normally discussed at an introductory level. Although they may initially appear to be complex, it is important to look for similarities; the individual steps are typically very simple and follow the same patterns as their shorter counterparts.
13.7.1. Reaction: Converting Esters to Carboxylic Acids (Hydrolysis/Saponification)
It is possible to convert an ester into a carboxylic acid using a hydroxide, typically sodium hydroxide (NaOH; Scheme 13.16). This is sometimes referred to as hydrolysis but is traditionally referred to as saponification; this is the process that occurs in traditional soap making when lye (a mixture of hydroxides) is mixed with animal fat (triglycerides, which contain three ester functional groups).
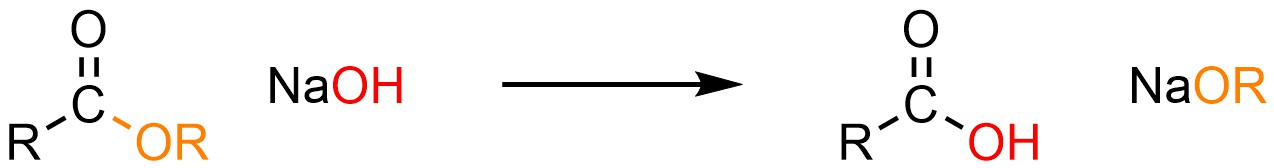
Scheme 13.16 – Generalized Reaction Equation for Addition-Elimination Converting Esters to Carboxylic Acids Using Hydroxide.
Esters and carboxylic acids are roughly equivalent in terms of electrophilicity. Because hydroxides (–OH) and alkoxides (–OR) are also roughly equivalent in terms of nucleophilicity the addition-elimination step is reversible (Scheme 13.17). However, the products are an acid (carboxylic acid) and a base (alkoxide). They react with each other to form a carboxylate and an alcohol. This step is irreversible and consumes the products, driving the equilibrium to the right (LeChatelier’s principle).

Scheme 13.17 – Generalized Reaction Equation for Addition-Elimination Converting Esters to Carboxylic Acids Using Hydroxide Showing Equilibrium and Reaction Quench.
The reaction must be quenched after this using a strong acid to generate the final product. The overall reaction is often depicted as two steps to indicate that this is required (Scheme 13.18).
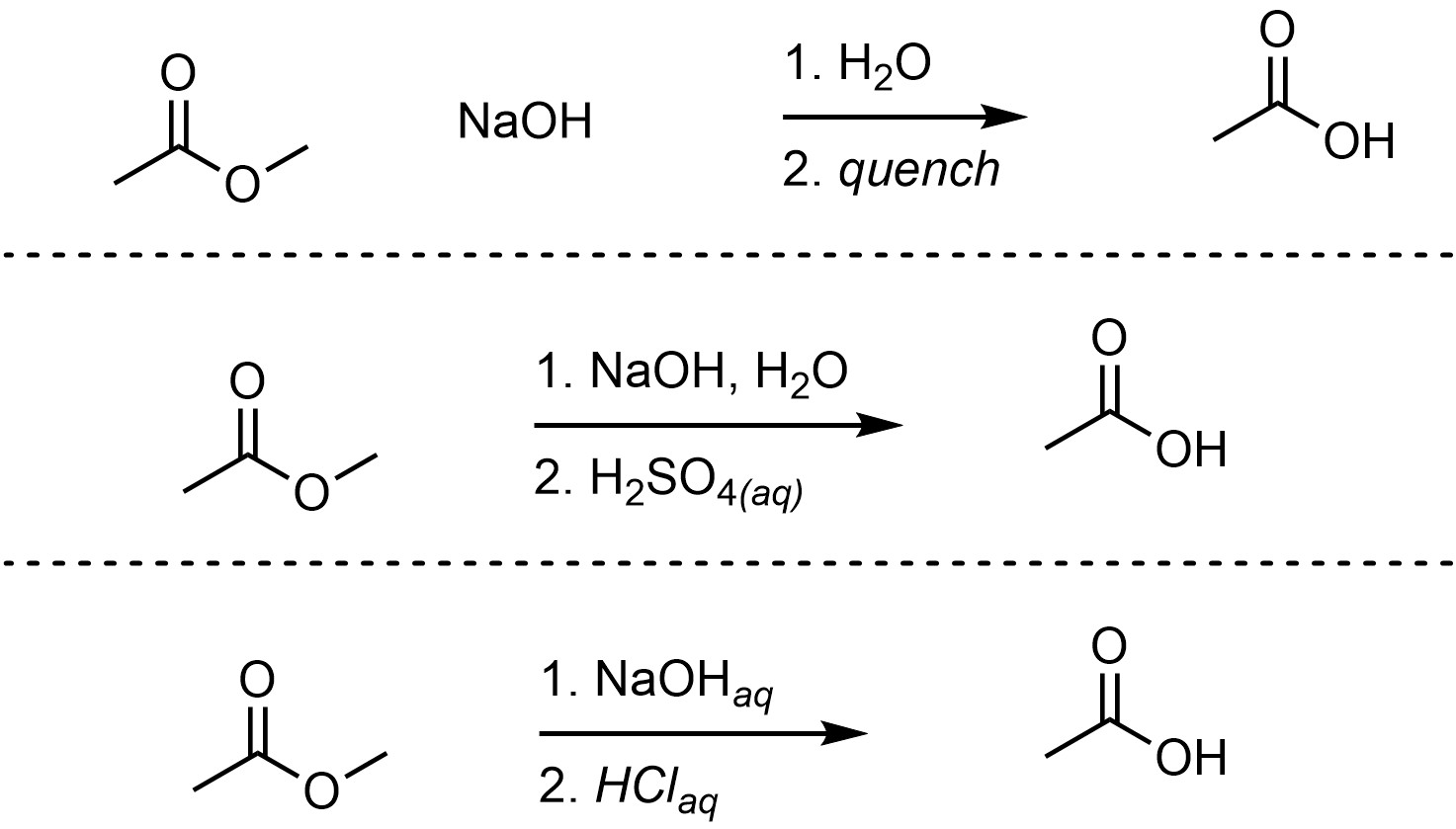
Scheme 13.18 – Examples of Ways of Depicting Overall Reactions for Saponification Reactions.
As a direct result of the acid-base reactivity of carboxylic acids and alkoxides, this approach can be used to convert an ester into a carboxylic acid but cannot be used to convert a carboxylic acid into an ester. If a carboxylic acid and an alkoxide are combined the simple acid-base reaction will occur instead (Scheme 13.19).
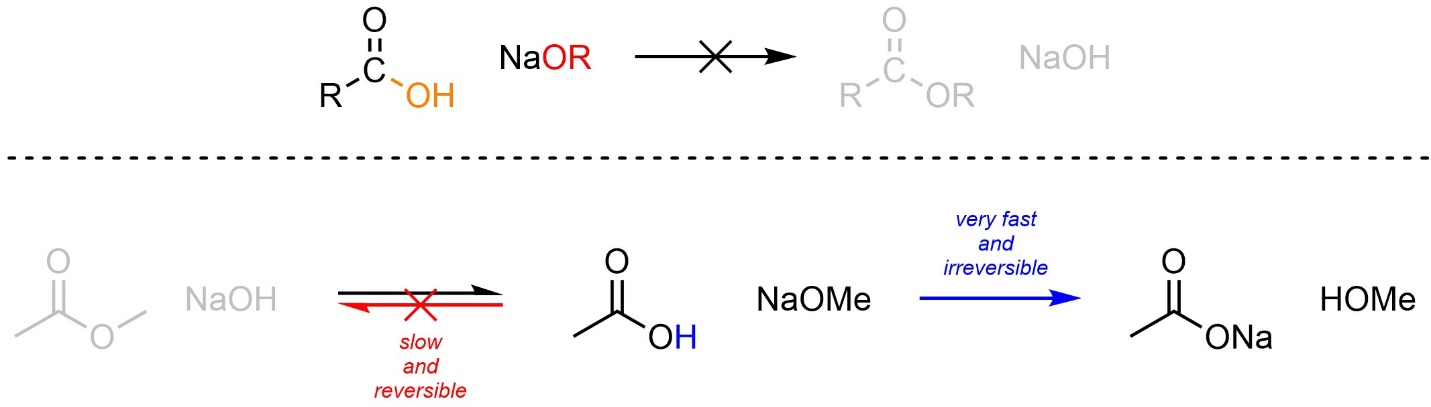
Scheme 13.19 – Example Highlighting Potential Reactions and Preferred Pathway When Combining a Carboxylic Acid and Alkoxides.
13.7.1.1. Mechanism
This reaction follows the standard two step mechanism for addition-elimination reactions (see Section 13.3) followed by a deprotonation and eventual quench (Scheme 13.20). The nucleophile (hydroxide) attacks the electrophile (ester). This forms a new bond and adds a lone pair to the oxygen. Then that lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group (alkoxide). The two products then react with each other; the base (alkoxide) removes a proton from the acid (carboxylic acid). After the reaction is complete a strong acid is added to quench, forming the final product.
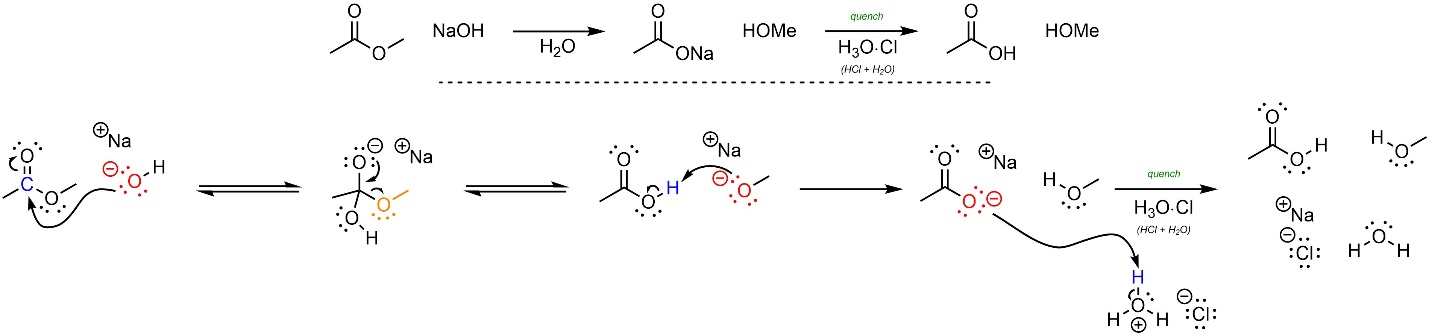
Scheme 13.20 – Reaction Mechanism for Addition-Elimination (Acylation) of Sodium Hydroxide with Methyl Ethanoate and Subsequent Quench with Hydrochloric Acid.
13.7.2. Reaction: Interconverting Carboxylic Acids and Esters (Fischer Esterification)
It is possible to convert an ester into a carboxylic acid, or a carboxylic acid into an ester, using an acid catalyst and water (H-OH) or an alcohol (H-OR; Scheme 13.21). The latter process is sometimes referred to as Fischer Esterification.
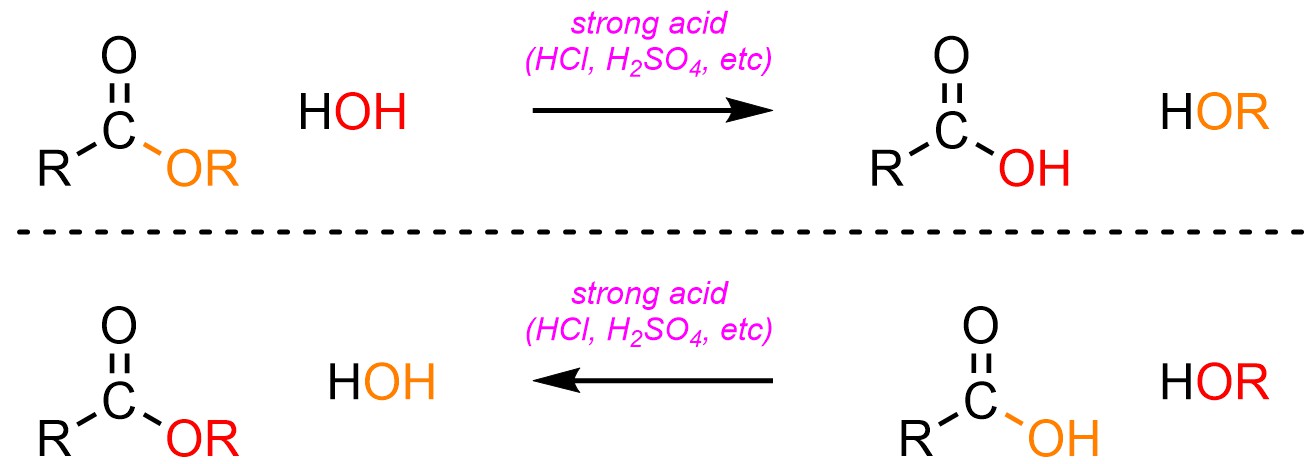
Scheme 13.21 – Generalized Reaction Equations for Addition-Elimination Converting Esters to Carboxylic Acids or Carboxylic Acids to Esters Using an Acid Catalyst.
Esters and carboxylic acids are roughly equivalent in terms of electrophilicity. Because water (H-OH) and alcohols (H-OR) are also roughly equivalent in terms of nucleophilicity the addition-elimination step is reversible. Equilibrium is controlled by the solvent: if the carboxylic acid is desired the solvent is water; if the ester is desired the solvent is the corresponding alcohol (Scheme 13.22). Because water and alcohol are starting materials/products adding a large excess of one (by using it as the solvent) will drive the equilibrium to the other side of the equation (LeChatelier’s principle).
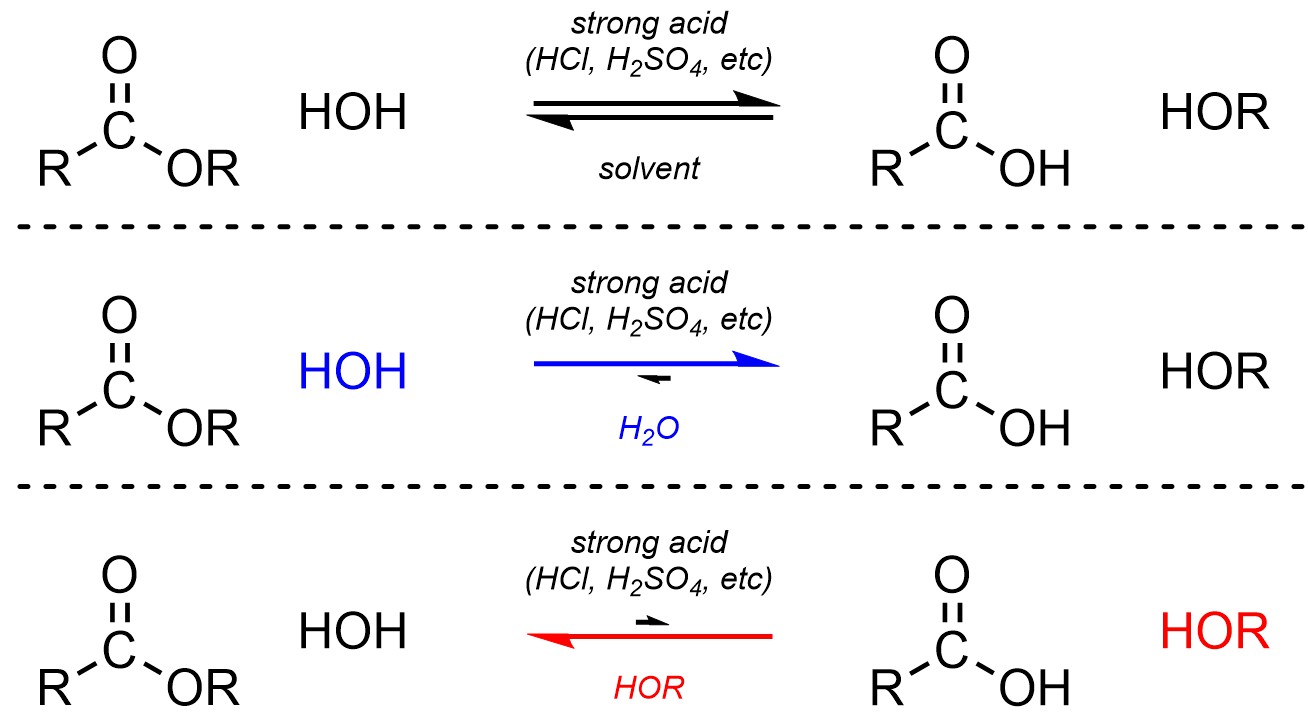
Scheme 13.22 – Generalized Reaction Equations for Addition-Elimination Converting Esters to Carboxylic Acids or Carboxylic Acids to Esters Using an Acid Catalyst.
The catalyst for the reaction must be a strong acid. It is possible to use acids such as sulfuric acid (H2SO4), phosphoric acid (H3PO4), or nitric acid (HNO3) but it is much more common to use hydrochloric acid (HCl). Like sulfuric acid, phosphoric and nitric acid always contain at least some water. If the desired product is an ester this can negatively affect the reaction. However, it is possible to generate hydrochloric acid without any water in it (sometimes referred to as anhydrous hydrochloric acid). How this is done is beyond the scope of the text.
13.7.2.1. Mechanism
The mechanisms for these reactions are identical, with only a single group being changed between them (Scheme 13.23). The strong acid reacts with water/alcohol to form hydronium (not shown). This is the active catalyst. First, the electrophile (carbonyl) gets activated by the catalyst. This greatly increases its electrophilicity. Second, the water/alcohol (nucleophile) attacks the activated carbonyl (electrophile). This forms a new bond and adds a lone pair to the oxygen. The solvent then removes a proton from one oxygen (the former nucleophile) and places it on the desired leaving group. This greatly increases its leaving group ability. These steps may be viewed as regeneration of the catalyst and another acid-catalyzed activation. Then the lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group (alcohol/water). Finally, water/alcohol removes a proton, generating the final product and regenerating the catalyst.
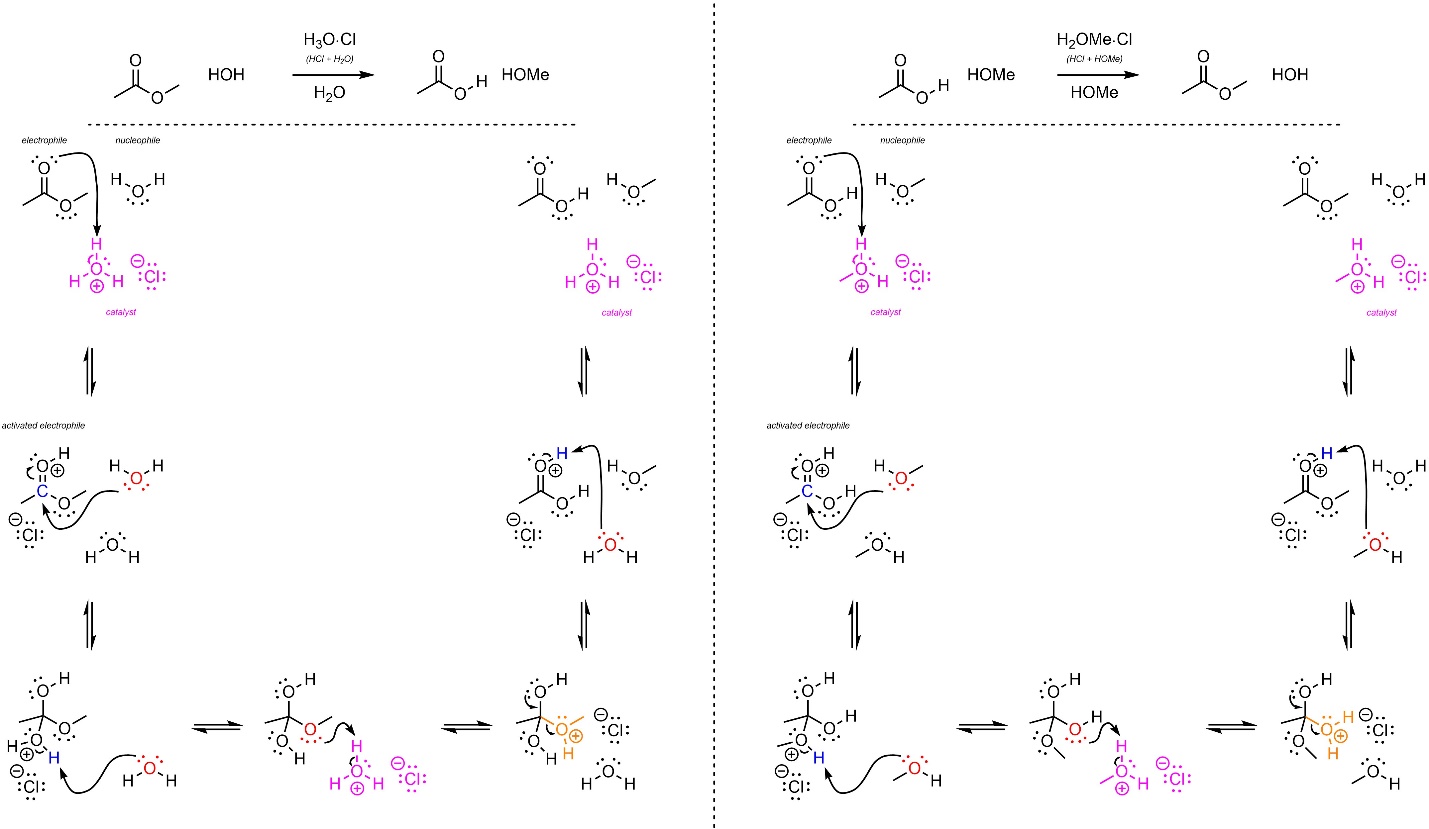
Scheme 13.23 – Reaction Mechanisms for Water Attacking Methyl Ethanoate and Methanol Attacking Ethanoic Acid with Acid Catalysis.
It may be useful to view these mechanisms as a series of three steps followed by the opposite three steps (Scheme 13.24). This is true for both mechanisms because they are identical.
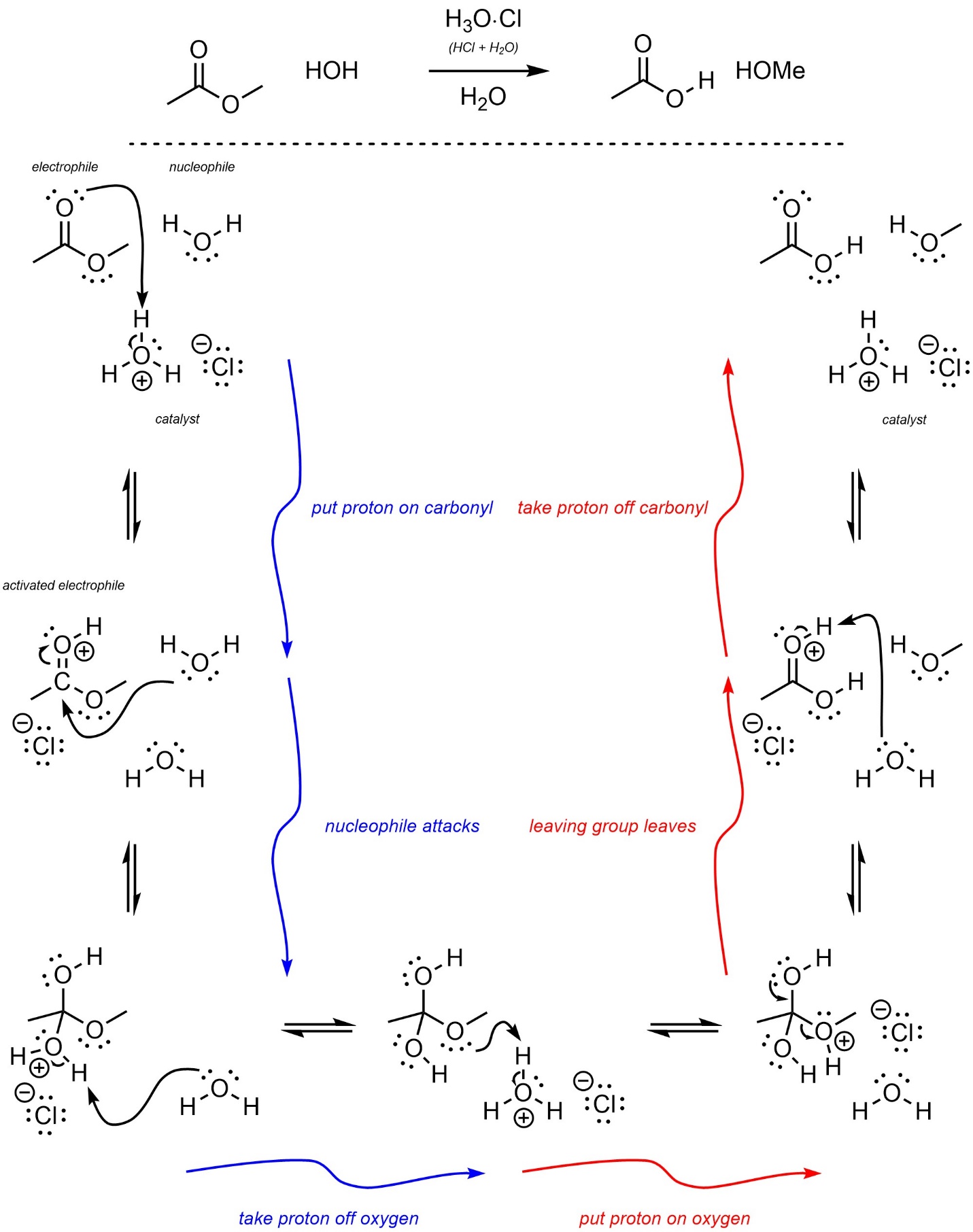
Scheme 13.24 – Highlight of Symmetry of Steps in Acid-Catalyzed Addition-Elimination Mechanisms.
13.7.3. Reaction: Interconverting Esters (Fischer Transesterification)
Using the same approach and rationale as for Hydrolysis/Fischer Esterification (see Section 13.8.2) it is possible to convert an ester into a different ester using an acid catalyst and an alcohol (H-OR; Scheme 13.25). This is sometimes referred to as Fischer Transesterification.
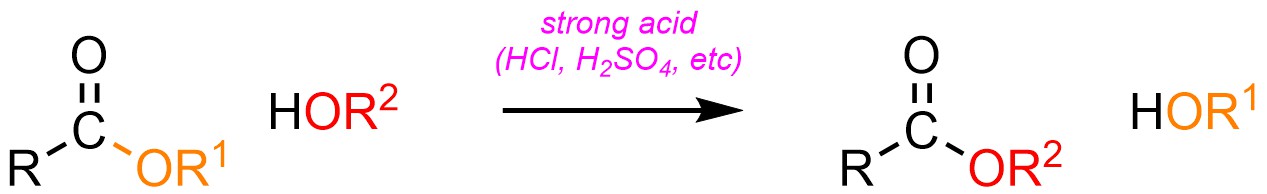
Scheme 13.25 – Generalized Reaction Equation for Addition-Elimination Converting an Ester to A Different Ester Using an Acid Catalyst.
13.7.3.1. Mechanism
The mechanism for this reaction is identical to that of Hydrolysis/Fischer Esterification (see Section 13.7.2.1; Scheme 13.26).
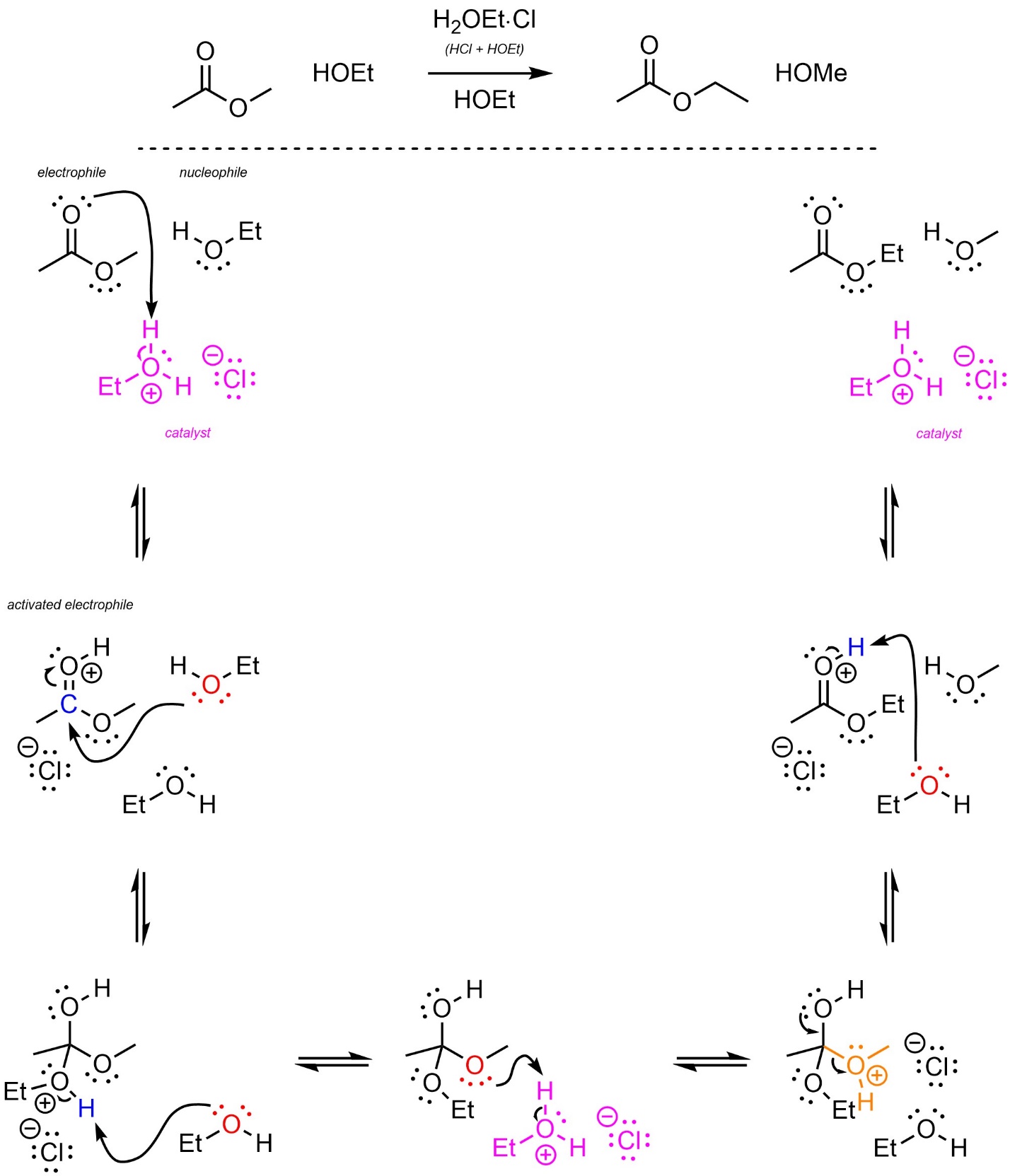
Scheme 13.26 – Reaction Mechanism for Ethanol Attacking Methyl Ethanoate with Acid Catalysis.
13.7.4. Reaction: Converting Carboxylic Acids to Acid Halides
It is possible to convert a carboxylic acid into an acid halide using one of several special reagents. By far the most common reagent used for these reactions is thionyl chloride (SOCl2; Scheme 13.27). Other reagents have different structures but follow the same general mechanism (see Section 13.7.4.1).
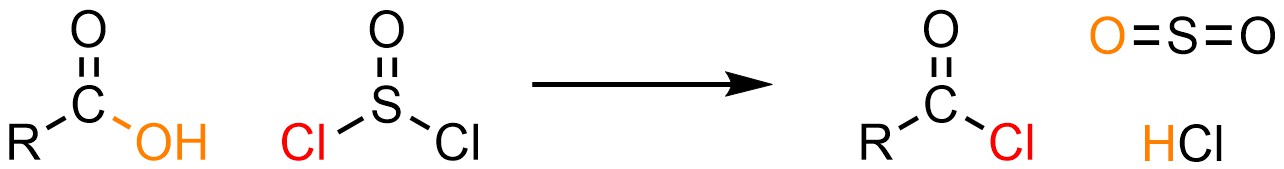
Scheme 13.27 – Generalized Reaction Equation for Addition-Elimination Converting Carboxylic Acids to Acid Chlorides Using Thionyl Chloride.
Thionyl chloride is highly reactive (very electrophilic) and consequently somewhat challenging to work with; it may react with moisture in the air, and any solvent that is moderately nucleophilic may not be used with it. It is possible to convert carboxylic acids into other acid halides (acid bromides, acid iodides) using this approach and reagents other than thionyl chloride. However, in practice these reactions are uncommon as the reagents required for them are usually even more reactive and very challenging to work with.
13.7.4.1. Mechanism
The mechanism for this reaction is more complex than unusual (Scheme 13.28). The first two steps are an addition-elimination reaction with thionyl chloride as the electrophile. This forms a halide and a strongly activated electrophilic carbonyl. Then the halide (nucleophile) attacks the activated carbonyl (electrophile). Although halides are not very nucleophilic the activated carbonyl is electrophilic enough for this to occur. The unusual nature of the intermediate created allows several things to occur at the same time: the electrons in the H-O σ bond become a new O-C π bond, the electrons in the other C-O σ bond become a new O-S π bond, and the electrons of the S-Cl σ bond become a new Cl-H σ bond. This generates the final product, sulfur dioxide, and hydrochloric acid.
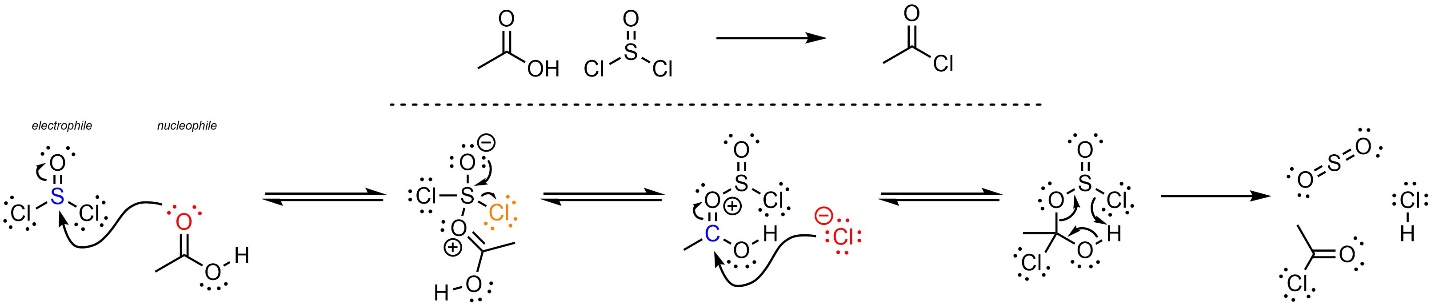
Scheme 13.28 – Reaction Mechanism for Addition-Elimination of Thionyl Chloride with Ethanoic Acid.
The final step is advanced for an introductory level and does not follow the typical patterns (acid-base, nucleophile-electrophile, leaving group, etc). It is sometimes referred to as a rearrangement step. These steps are common in many complex reactions/mechanisms and are discussed in more detail in advanced texts. At an introductory level understanding the details of this step (not discussed) is not required, but being able to replicate it is.
13.7.5. Reaction: Converting Carboxylic Acids/Esters to Amides
It is technically possible to convert a carboxylic acid or ester into an amide using an amine (H-NR2; Scheme 13.29).
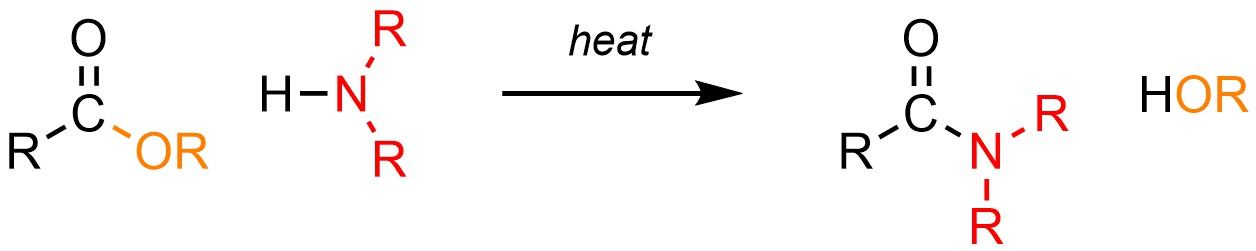
Scheme 13.29 – Generalized Reaction Equation for Addition-Elimination Converting Carboxylic Acids or Esters to Amides.
These reactions follow the typical mechanism for addition-elimination (see Section 13.3). However, because amines are more basic than nucleophilic and carboxylic acids/esters are only moderately electrophilic, these reactions are very slow and require extreme conditions; typically the reaction takes several days or weeks at boiling or supercritical temperatures to generate a moderate amount of product. As a result these reactions are very uncommon, with most chemists preferring to use an alternative approach (see Section 13.7.5.1).
13.7.5.1. Solving Problems Using Special Reagents – DCC
It is possible to convert a carboxylic acid into an amide using an amine (H-NR2) and one of several special reagents. By far the most common reagent used for these reactions is dicyclohexylcarbodiimide (DCC; Scheme 13.30). Other reagents have different structures but follow the same general mechanism (see Sections 13.7.5.2 and 13.7.5.3).
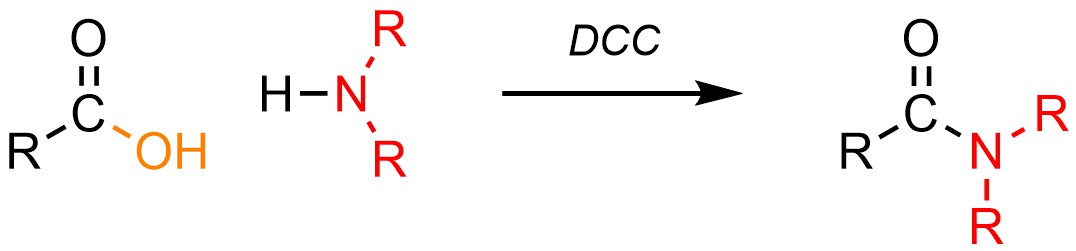
Scheme 13.30 – Generalized Reaction Equation for Converting Carboxylic Acids to Amides Using Dicyclohexylcarbodiimide (DCC).
13.7.5.2. Mechanism
The mechanism for this reaction is significantly more complex than unusual (Scheme 13.31). The unusual nature of DCC allows several things to occur at the same time in the first step: the electrons in the H-O σ bond become a new O-C π bond, the electrons in the other C-O π bond become a new O-C σ bond, and the electrons of the C-N π bond become a new N-H σ bond. It is often useful to re-orient this intermediate to better understand the next step; the intermediate formed is structurally similar to an anhydride, which makes it significantly more electrophilic than a carboxylic acid. Then the amine (nucleophile) attacks the activated carbonyl (electrophile). This intermediate is again able to have several things occur at the same time: the lone pair comes back and reforms the π bond with the carbon, the electrons in the other C-O σ bond become a new O-C π bond, the electrons in the C-N π bond become a new N-H σ bond, and the electrons of the H-C σ bond become a lone pair on the nitrogen. This single step is equivalent to an elimination occurring at the same time as the deprotonation that normally follows (see Section 13.6.2). This generates the final product and a urea.
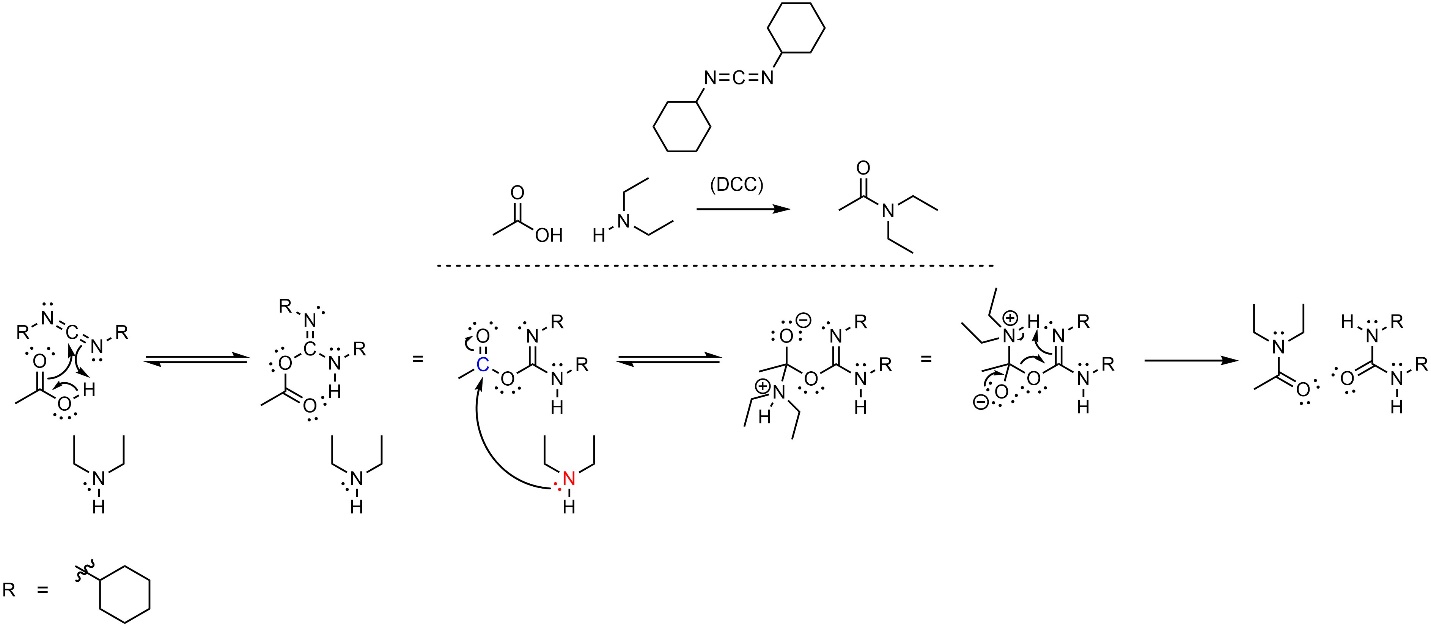
Scheme 13.31 – Reaction Mechanism for Combining Ethanoic Acid and Diethylamine Using Dicyclohexylcarbodiimide (DCC).
The first and final steps are advanced for an introductory level and do not follow the typical patterns (acid-base, nucleophile-electrophile, leaving group, etc). These are again rearrangement steps (see Section 13.7.4.1). At an introductory level understanding the details of these steps (not discussed) is not required, but being able to replicate them is.
13.7.5.3. Alternatives to DCC
The cyclohexyl groups of DCC do not participate in the mechanism (see Scheme 13.31). The most common alternatives to DCC simply change those groups (Figure 13.6). The primary reason for these alternatives is to adjust solubility; some carboxylic acids and/or amines require the use of specific solvents in which DCC may not be soluble. At an introductory level learning/memorizing the specific conditions where these may be required is not necessary. It is important only to understand that there are alternatives that follow the same mechanism.
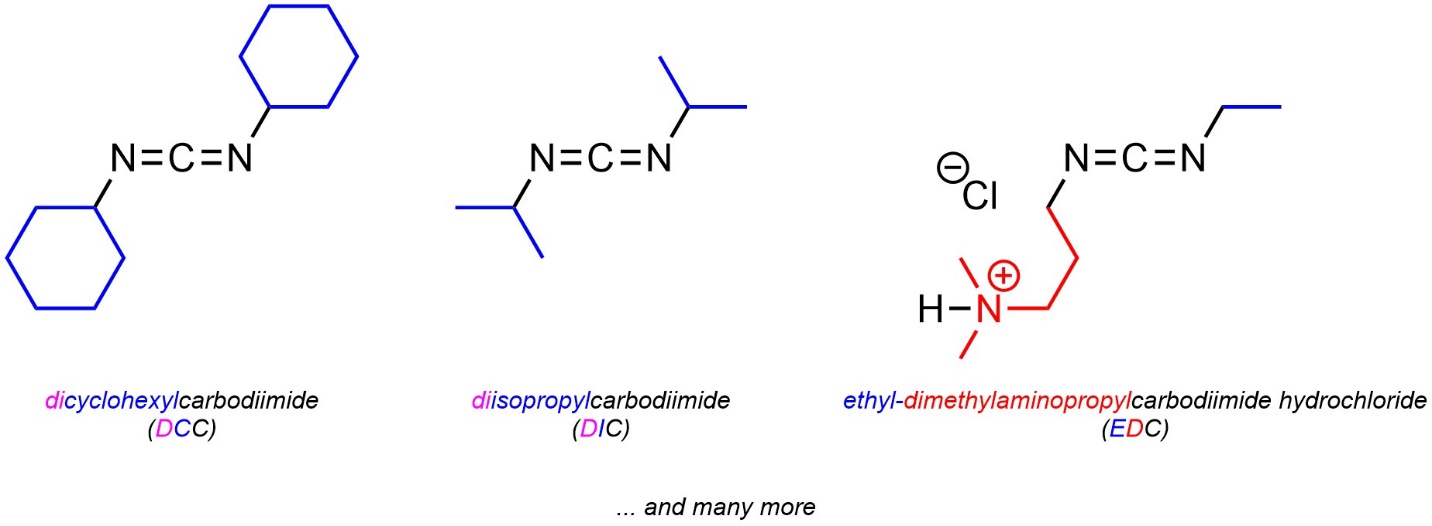
Figure 13.6 – Examples of Carbodiimides Used for Synthesis of Amides from Carboxylic Acids and Amines.

