8.3. Regioselectivity
Instead of a single nucleophilic atom, π bonds represent a group of atoms which is overall nucleophilic. This introduces a new complication to reactions. Consider a non-symmetrical alkene adding the generalized X-Y across the double bond (Scheme 8.2). There are two different compounds possible as products.
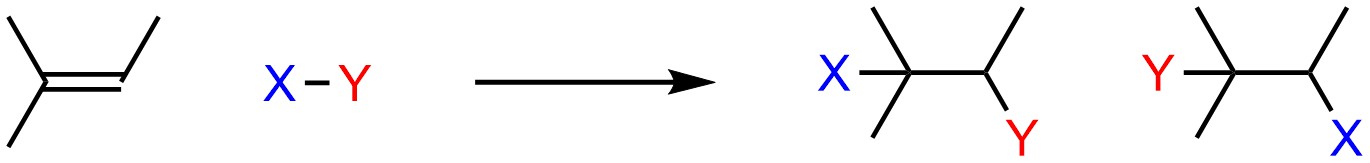
Scheme 8.2 – Regioisomers from Electrophilic Addition of X-Y to 2-Methylbut-2-ene.
The relationship between these two products is constitutional isomers. When multiple constitutional isomers are possible products of a reaction, they are referred to as regioisomers (constitutional isomers that result from different atoms/groups going to different regions of the product).
For many reactions multiple regioisomers are possible. If the regioisomers are not formed in equal amounts, the reaction is described as being regioselective (selectively forming more of one regioisomer over the other(s)). A complete understanding of how regioselectivity can be controlled or achieved lies beyond the scope of this text. Instead, a brief explanation is sufficient to provide context.
Regioselectivity can be achieved if the reaction is fully reversible and there is a moderate to large energy difference between the products (the constitutional isomers). This is usually referred to as thermodynamic control because the control comes from being able to reach thermodynamic equilibrium. This type of regioselectivity is not discussed in this text.
Regioselectivity can be achieved if there is a moderate to large energy difference between transition states leading to the two products. This is usually referred to as kinetic control because the control comes from one pathway being kinetically faster than the other(s).

