13.9. Multiple Additions of Organometallic Reagents to Acyl Compounds
Recall that organometallics such as Grignard and organolithium reagents are both strong nucleophiles and bases (see Section 7.6). Depending on which organometallic is chosen there can be a moderate difference in nucleophilicity. For example, organolithium reagents (R-Li) are more nucleophilic than Grignard reagents (R-MgX). Organometallics can react as nucleophiles with electrophilic carbonyls. Mechanisms for nucleophilic attacks of organometallic compounds onto electrophiles are, in reality, incredibly complex. While the specifics of the reaction mechanism are complex, they can be approximated as simple nucleophile-electrophile attacks. Finally, recall that these reactions must be quenched using water and/or a weak acid to break the O-Li or O-MgX bonds and generate the final product. The overall reactions are often depicted as two steps to indicate that this is required.
Organometallics, including Grignard and organolithium reagents, can attack carbonyl-containing functional groups other than aldehydes and ketones. This follows the standard mechanism and pattern for addition-elimination reactions (see Sections 13.2 and 13.3). After the addition-elimination reaction the product is a new carbonyl-containing functional group (Scheme 13.41). Another organometallic may attack this product. This follows the standard mechanism and pattern for nucleophilic attacks of organometallic reagents on carbonyls (see Section 7.6).
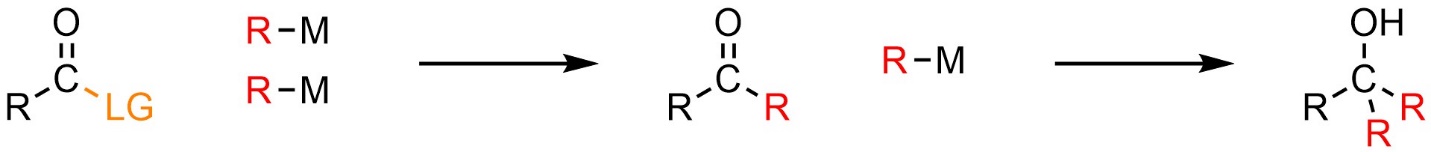
Scheme 13.41 – Generalized Reaction Equation for Addition-Elimination Followed by Addition When Using a Nucleophilic Organometallic Reagent.
In theory these reactions can be performed using exactly two equivalents of the organometallic compound. In practice it is often challenging to accurately measure the precise amount of organometallic reagent being added. As a result, it is common to simply use an excess amount of the organometallic compound; the number of equivalents added is not important as long as it is at least two.
13.9.1. Issues with Carboxylic Acids
It is possible to have addition-elimination-like reactions between nucleophilic organometallic reagents and carboxylic acids. As with halide reductions of carboxylic acids the first step is an acid-base reaction (see Section 13.8.2, Scheme 13.34). Because there is no way to increase the leaving group ability of the anionic oxygen after this step, reactions between organometallic reagents and carboxylic acids undergo a different mechanism and produce a different product. However, the same outcome can be achieved much more reliably using a class of amides designed for this purpose (see Section 13.9.3). As a result, these reactions are very uncommon and will be omitted from this text.
13.9.2. Reactions with Acid Halides, Anhydrides, Esters, and Amides
It is possible to convert an acid halide, anhydride, ester, or amide into a tertiary alcohol using a moderate or strong nucleophilic organometallic reagent, typically a Grignard reagent (R-MgX) or organolithium (R-Li; Scheme 13.42). The solvent used must match the organometallic source (see Section 7.6.1), though often diethyl ether (Et2O) is used in either case. The reaction must be quenched using water and/or a weak acid to generate the final product.
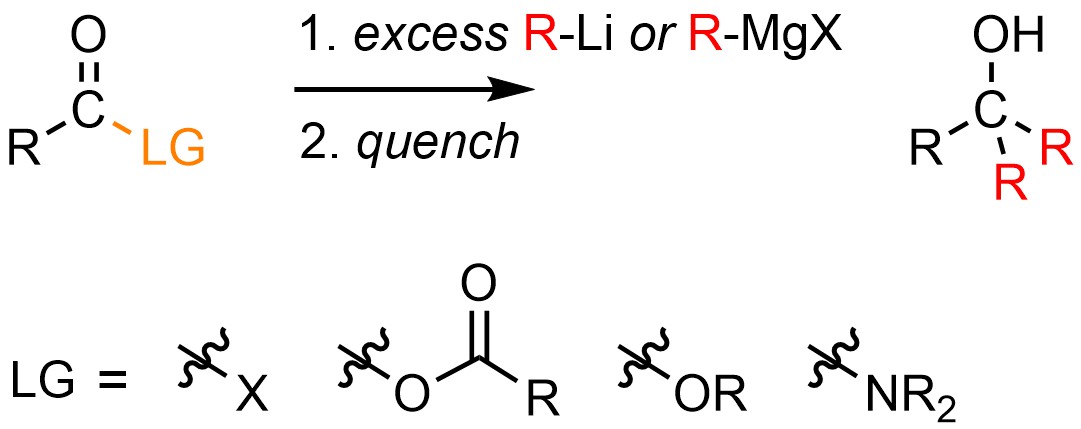
Scheme 13.42 – Generalized Reaction Equation for Converting Acid Halides, Anhydrides, Esters, or Amides to Tertiary Alcohols Using Grignard or Organolithium Reagents.
In reality there are many potential limitations and issues with these reactions. For example, using acid halides and anhydrides for these reactions is uncommon because they often undergo competitive side reactions instead of the desired addition-elimination addition. At an introductory level it is impossible to predict whether a given reaction will experience these issues. This text will specify when students should consider the possibility of limitations or issues during these reactions. In all other cases, assume the reaction will work as desired.
The mechanism for these reactions follows the standard addition-elimination mechanism followed by a standard addition mechanism (Scheme 13.43). The reactivity is approximated by assuming the organometallic behaves as a carbanion. First, a carbanion (nucleophile) attacks the carbonyl (electrophile). This forms a new bond and adds a lone pair to the oxygen. Then the lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group. A second carbanion (nucleophile) attacks the new carbonyl (electrophile). After the reaction is complete water and/or a weak acid is added to quench, forming the final product.
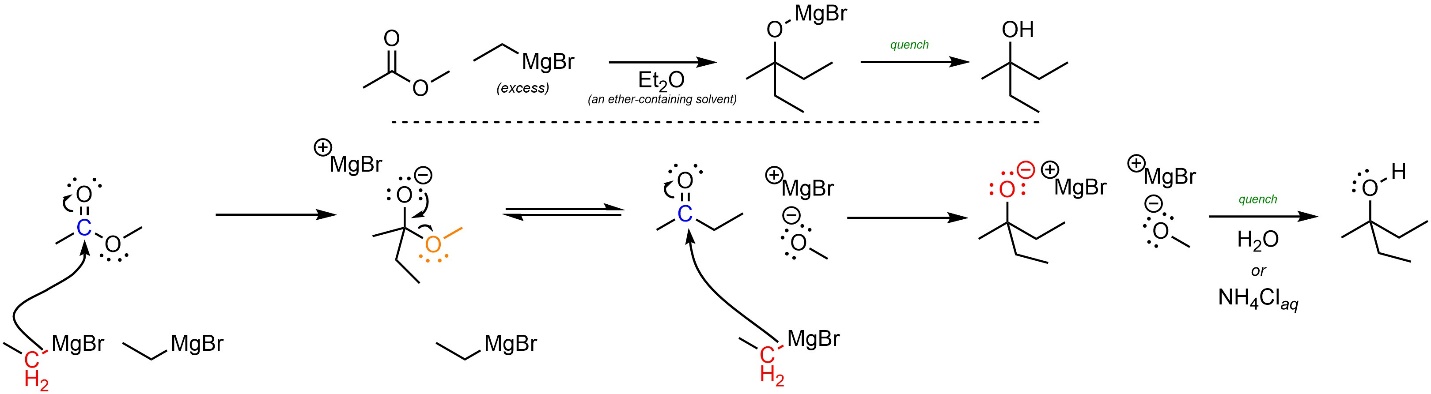
Scheme 13.43 – Reaction Mechanism for Addition-Elimination Followed by Addition of Methyl ethanoate with Ethylmagnesium bromide.
13.9.3. Solving Problems Using Special Electrophiles – Weinreb Amides
It is possible to convert a special kind of amide into a ketone using a moderate or strong nucleophilic organometallic reagent, typically a Grignard reagent (R-MgX) or organolithium (R-Li; Scheme 13.44). These amides have an oxygen-containing substituent on the nitrogen and are sometimes referred to as Weinreb Amides. The solvent used must match the organometallic source (see Section 7.6.1), though often diethyl ether (Et2O) is used in either case. The reaction must be quenched using a weak acid to generate the final product.
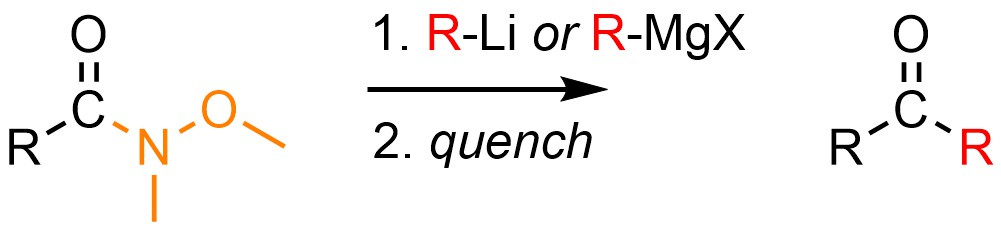
Scheme 13.44 – Generalized Reaction Equation for Converting Weinreb Amides to Ketones Using Grignard or Organolithium Reagents.
Weinreb amides work by exploiting coordination (sometimes called chelation). An in-depth discussion of coordination is beyond the scope of this text and is instead covered in introductory inorganic sources. The intermediate formed after the first nucleophilic attack has two atoms able to donate electron density to the metal (see Scheme 13.45). More importantly, this forms a (relatively) stable five-membered ring. Because re-forming the π bond and ejecting the leaving group would destroy the stable five-membered ring this step has a (much) higher activation energy than usual. As a result, the leaving group cannot leave until the five-membered ring is broken during the quenching step. The net effect is that the new carbonyl is not formed until after all of the nucleophile is removed (during the quench) so it cannot undergo a second addition reaction.
Coordination/Chelation is often represented by drawing dotted lines between the atoms involved. At an introductory level this is encouraged but not required; drawing standard lines representing covalent bonds is acceptable.
The mechanism for these reactions is similar to the standard addition-elimination mechanism (Scheme 13.45). The reactivity is approximated by assuming the organometallic behaves as a carbanion. First, a carbanion (nucleophile) attacks the carbonyl (electrophile). This forms a new bond and adds a lone pair to the oxygen. Lone pairs from both oxygen atoms coordinate/chelate with the metal atom. This forms a semi-stable five-membered ring. After the reaction is complete water and/or a weak acid is added to quench. Then the lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group and forming the final product.
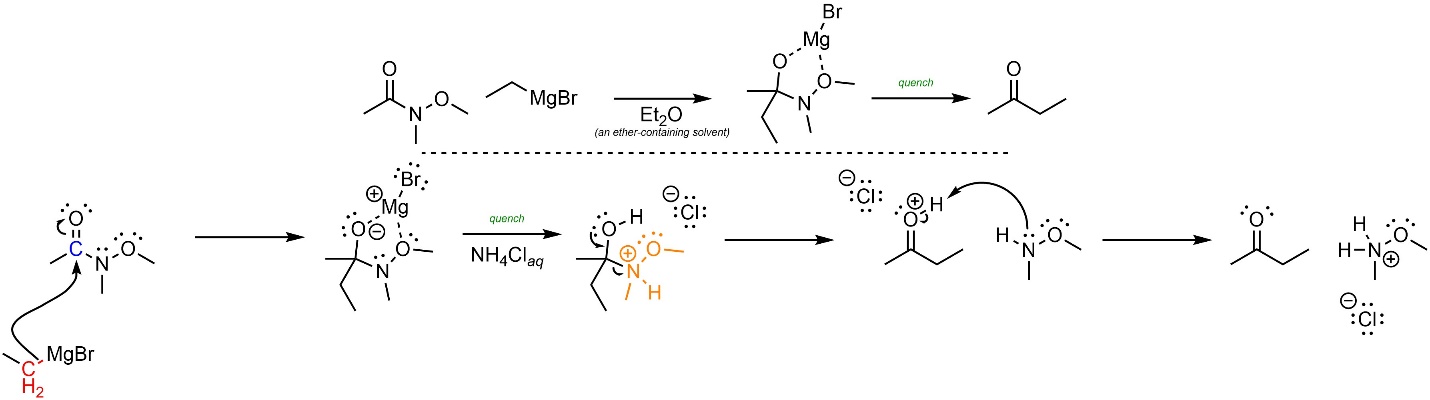
Scheme 13.45 – Reaction Mechanism for Addition-Elimination of N-Methoxy-N-methylethanamide with Ethylmagnesium bromide.

