9.3. Practical Considerations – Achieving Aromaticity and Avoiding Anti-Aromaticity
Being aromatic grants aromatic stabilization, which creates a very high degree of stability. As a result, if a molecule (or part of a molecule) can meet the conditions for aromaticity, it will. Being anti-aromatic has a large energy cost associated with it, which creates a very high degree of instability. As a result, if a molecule (or part of a molecule) can avoid meeting the conditions for anti-aromaticity, it will.
9.3.1. Aromatic Heterocycles (Heteroaromatic Compounds)
The atoms in an aromatic ring do not have to be carbons. For example, rings such as pyridine and pyrimidine are aromatic (Figure 9.7). It is important to remember that the hybridization on the nitrogen atoms is sp2; the lone pairs on nitrogen are in sp2 orbitals and therefore orthogonal to the π system. As a result, they are not in the π systems of the rings.
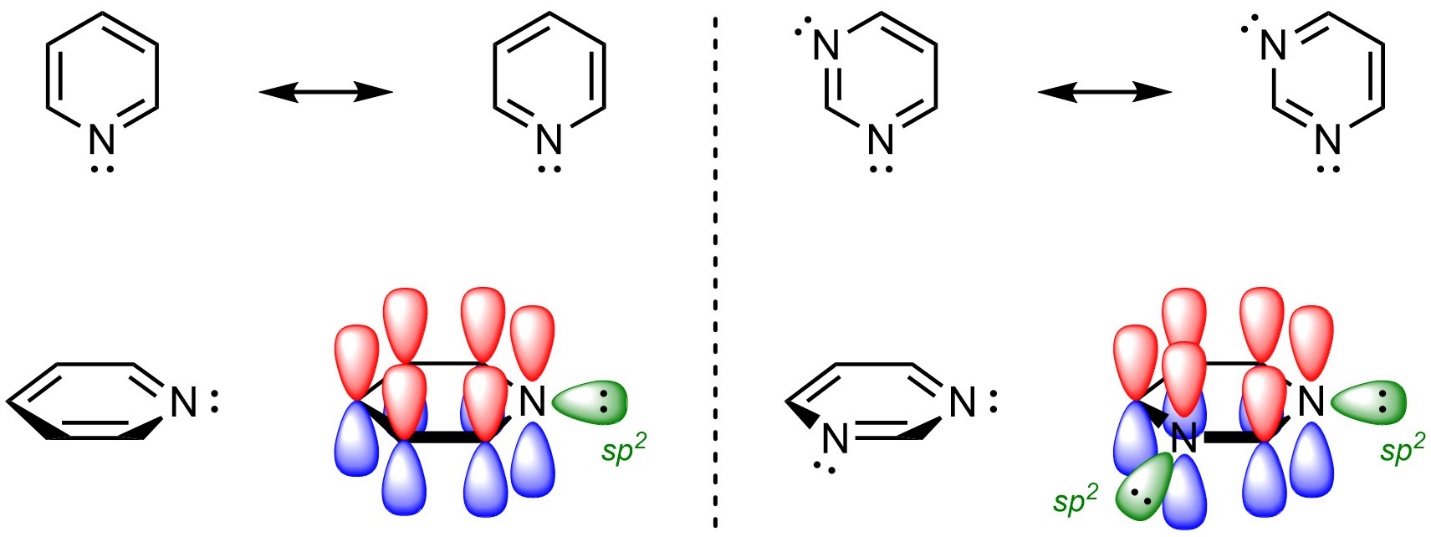
Figure 9.7 – Highlighting the Location of Electrons in Pyridine and Pyrimidine.
The electrons in the π system of an aromatic ring do not have to come from π bonds, they need only be in an overlapping (non-orthogonal) p orbital. Lone pairs on heteroatoms can contribute to the π system and help satisfy Hückel’s rule (Figure 9.8).
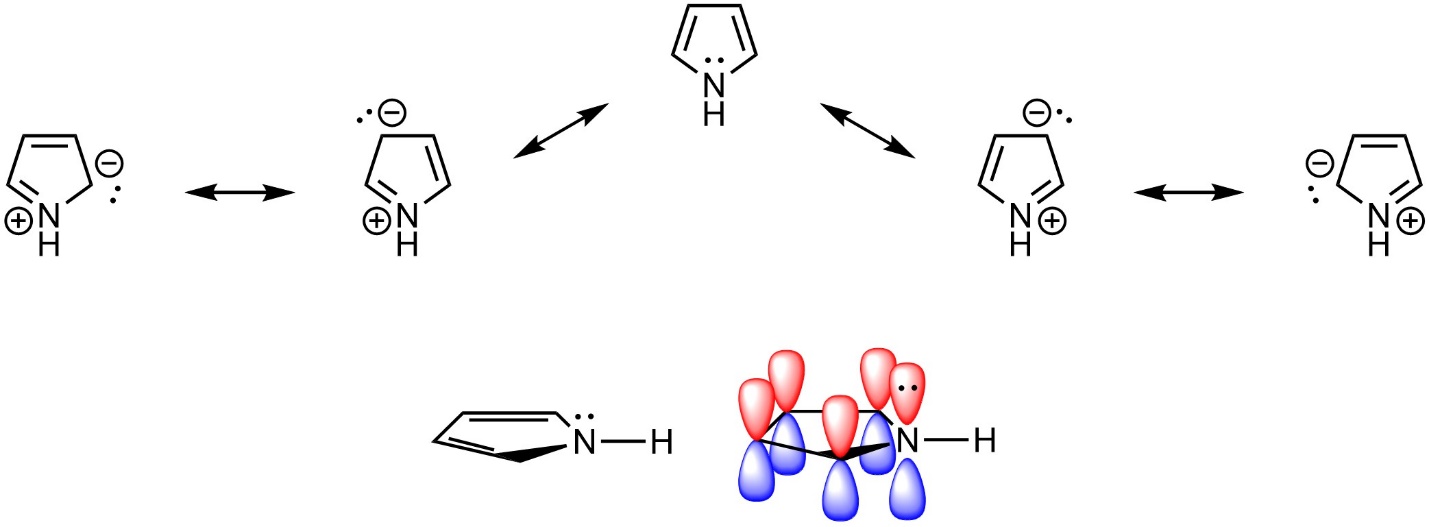
Figure 9.8 – Highlighting the Location of Electrons in Pyrrole.
It is often extremely helpful to draw out resonance structures to better identify aromaticity. This allows proper assignment of hybridization (see Section 5.5.7.). For example, there are two lone pairs on the oxygen of furan (Figure 9.9). At first glance, it may appear that furan is non-aromatic (with an sp3 hybridized oxygen) or anti-aromatic with 8 electrons in the π system of the ring. However, because the oxygen is properly assigned as sp2 hybridized, one lone pair is in the π system (by being in a p orbital) and one is in an orthogonal sp2 orbital. This makes furan aromatic.
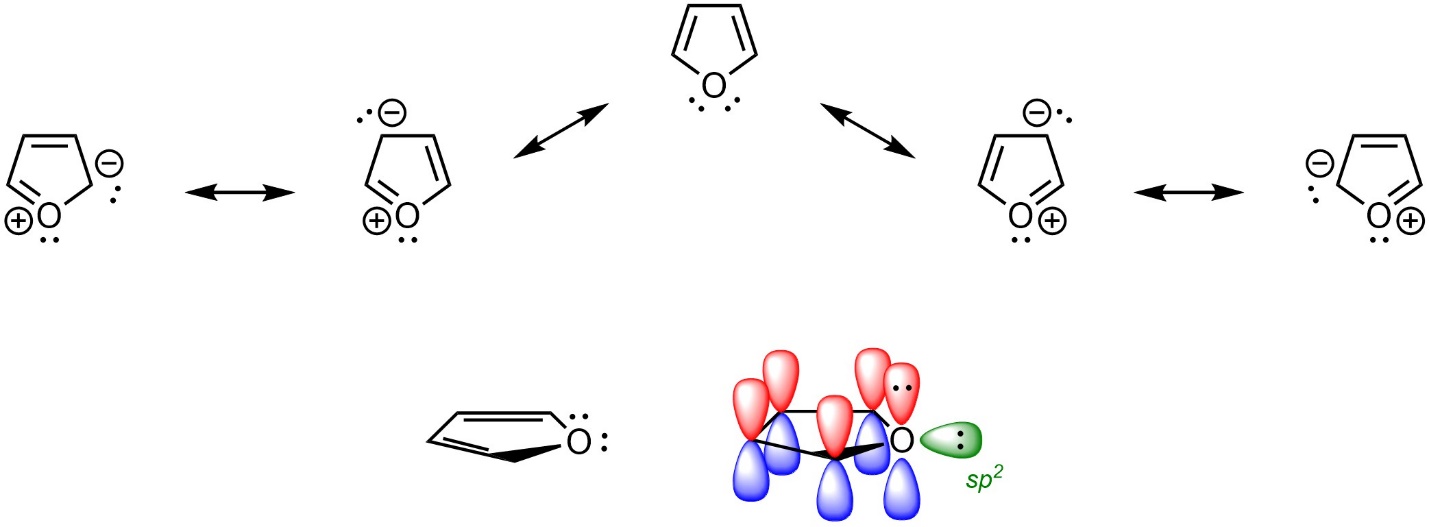
Figure 9.9 – Highlighting the Location of Electrons in Furan.
Aromatic rings with one or more heteroatoms in them are sometimes referred to as heteroaromatic rings. This distinction is not required; referring to them as aromatic rings is also correct.
9.3.2. Cationic/Anionic Aromatic Compounds
The electrons in the π system of an aromatic ring do not have to come from π bonds, they need only be in an overlapping (non-orthogonal) p orbital. Lone pairs on anions can contribute to the π system and help satisfy Hückel’s rule (Figure 9.10). For example, cyclopentadiene is not aromatic, but the deprotonated anionic cyclopentadienyl anion is.
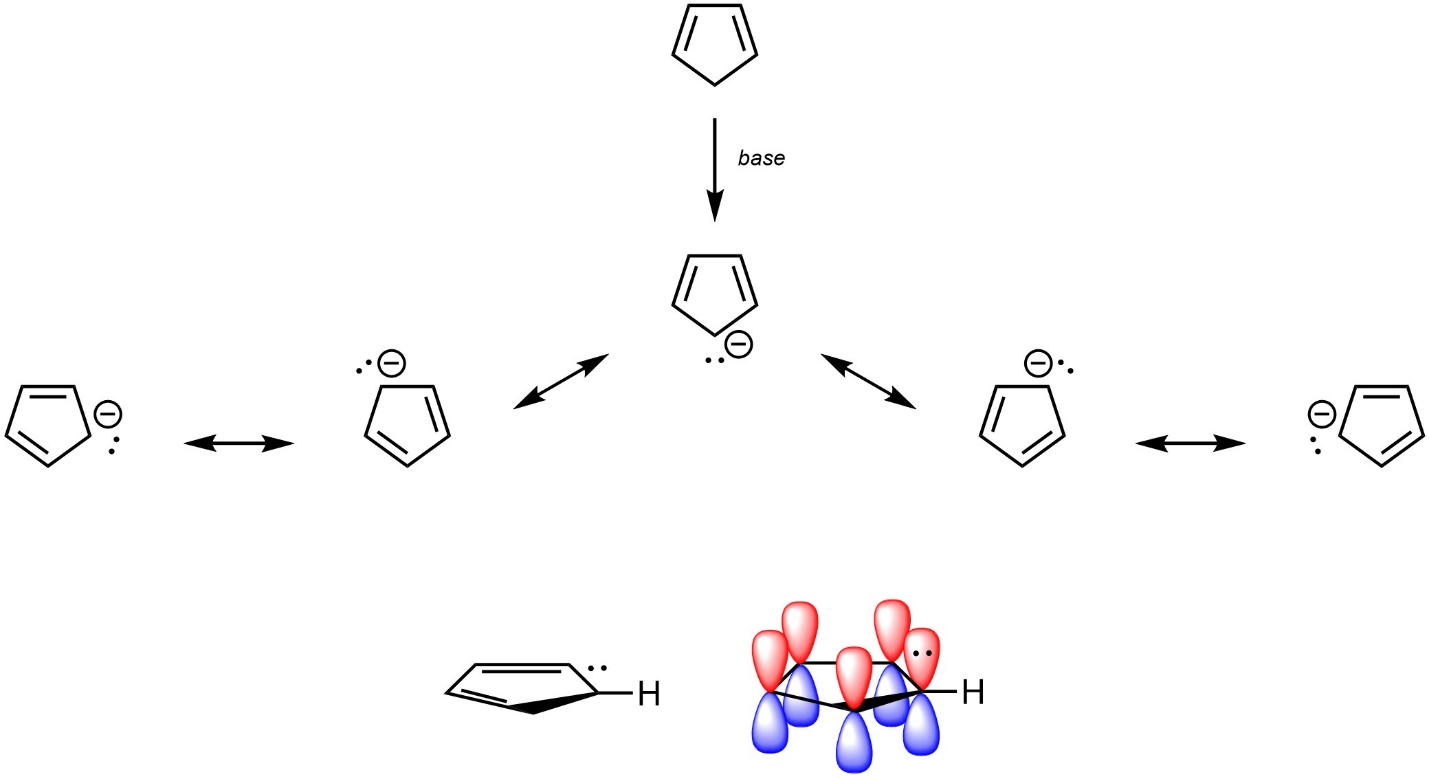
Figure 9.10 – Highlighting the Location of Electrons in Cyclopentadienyl Anion.
Conversely, the p orbitals on all atoms in the ring do not need to be filled. Carbocations are sp2 hybridized, with the p orbital being unoccupied. For example, the tropylium ion (carbocationic cycloheptatriene) is aromatic as a result of the overlap between its vacant p orbital with the others in the π system (Figure 9.11).
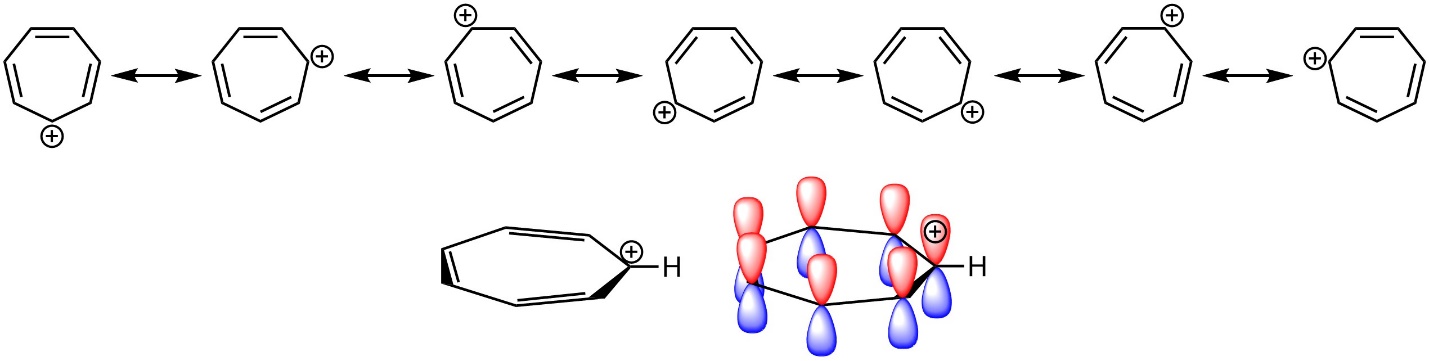
Figure 9.11 – Highlighting the Location of Electrons in Tropylium Cation.
9.3.3. Substituents and Aromaticity
Hückel’s rule states that the number of π electrons in the ring system must be a solution to [4n+2]. Substituents can interact with the π system of aromatic rings, but are not counted towards them for Hückel’s Rule. As a result, aromatic rings may exist with additional π electrons conjugated to the ring system (Figure 9.12). This does not affect aromaticity, but can have additional affects on chemical reactivity (see Section 10.10).
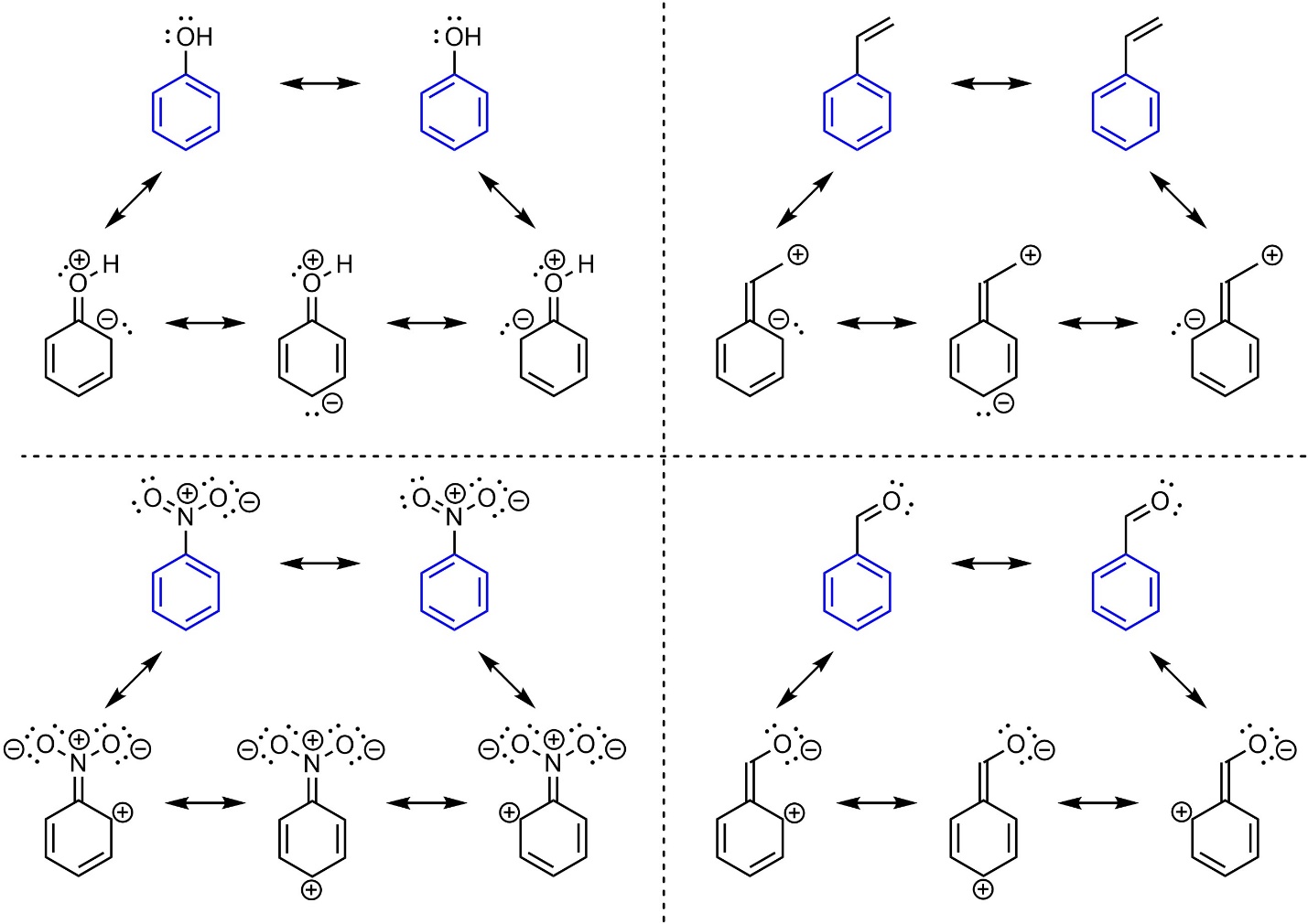
Figure 9.12 – Examples of Aromatic Rings with Conjugated Substituents.
Aromatic rings may be directly attached (fused) to other rings, including other aromatic rings (Figure 9.13). Fused aromatic rings are viewed as larger aromatic systems; instead of multiple aromatic rings they are considered a single large aromatic system. In some instances the aromatic system can become exceptionally large (see Figure 4.29), even deviating slightly from planarity.
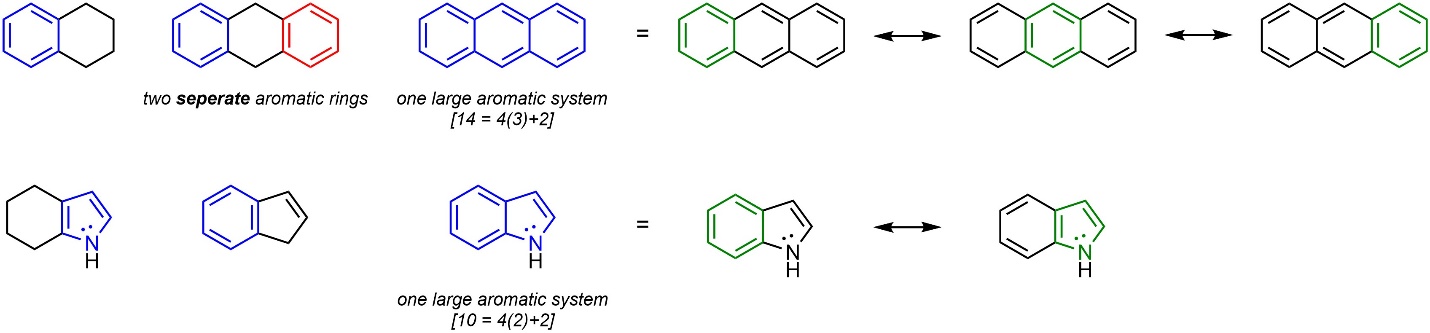
Figure 9.13 – Examples of Aromatic Rings with Substituents and Fused Aromatic Systems.
9.3.4. Conformational Changes
Being aromatic grants aromatic stabilization, which creates a very high degree of stability. As a result, if a molecule (or part of a molecule) can meet the conditions for aromaticity, it will. This includes conformational changes, if possible. However, it is not always geometrically possible to adopt a conformation that would allow aromaticity. A simple example is cyclodeca-1,3,5,7,9-pentaene (sometimes called [10]-annulene). This molecule can exist as two stereoisomers (Z, Z, Z, Z, Z; E, Z, Z, E, Z). When drawn two dimensionally, both isomers appear to be aromatic (Figure 9.14). However, it is not geometrically possible for either of these isomers to be planar.
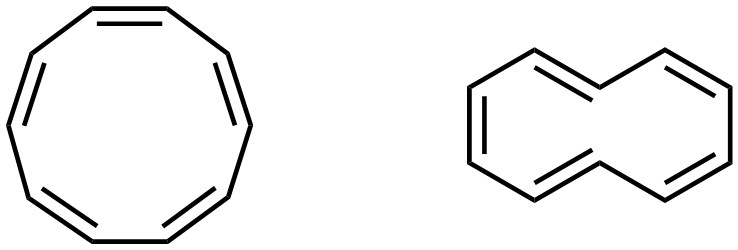
Figure 9.14 – Two Stereoisomers of Cyclodeca-1,3,5,7,9-pentaene.
The internal angles of a regular decagon are 144°. As a result, adopting a planar conformation for the first isomer would allow it to gain aromaticity but would introduce an excessive amount of angle strain at every position in the ring. The net result is that this isomer instead bends and adopts a bowl-like conformation (Figure 9.15). Because it is not planar, it is non-aromatic instead of aromatic.
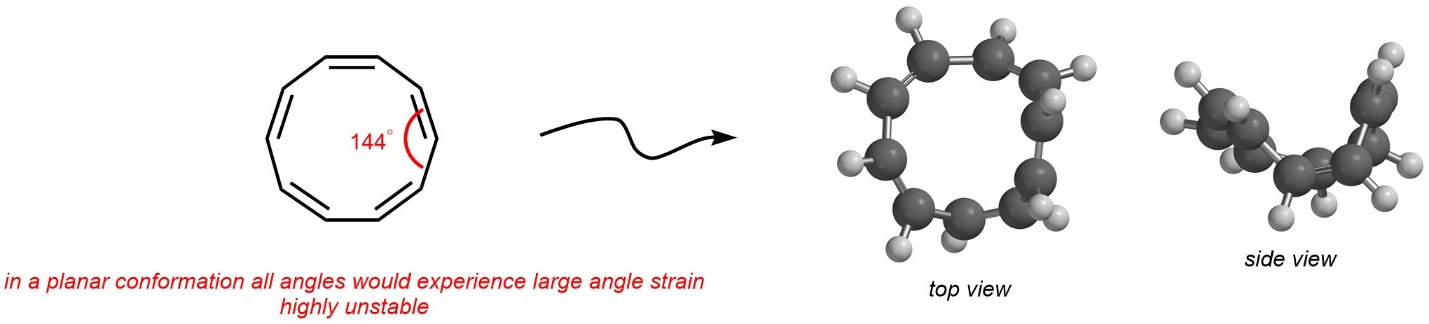
Figure 9.15 – Angle Strain Affecting the Conformation of Z,Z,Z,Z,Z-Cyclo-1,3,5,7,9-pentaene.
Hydrogens are omitted from Line-Angle structures. Consider the two internal hydrogens of the other isomer (Figure 9.16). A planar conformation is not possible because these two hydrogens would have to physically occupy the same space. The net result is that this isomer instead bends out of planarity. Because it is not planar, it is non-aromatic instead of aromatic.
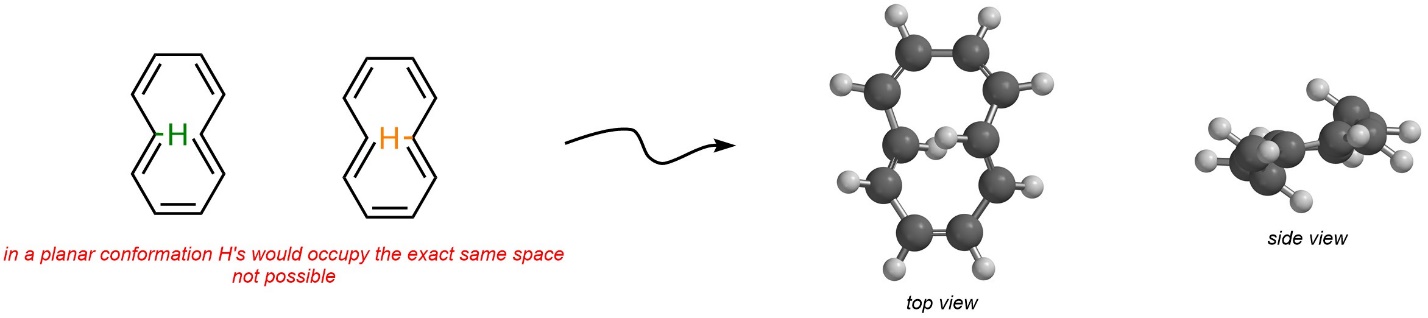
Figure 9.16 – Steric Strain Affecting the Conformation of E,Z,Z,E,Z-Cyclo-1,3,5,7,9-pentaene.
Being anti-aromatic has a large energy cost associated with it, which creates a very high degree of instability. As a result, if a molecule (or part of a molecule) can avoid meeting the conditions for anti-aromaticity, it will. This includes conformational changes, if possible. A simple example is cycloocta-1,3,5,7-tetraene (sometimes called [8]-annulene). When drawn two dimensionally, it appears to be anti-aromatic (Figure 9.17). However, it is geometrically possible for it to change conformation and avoid being planar. The result is that this compound adopts a bent conformation with only minimal angle and torsional strains. Because it is not planar, it is non-aromatic instead of anti-aromatic.
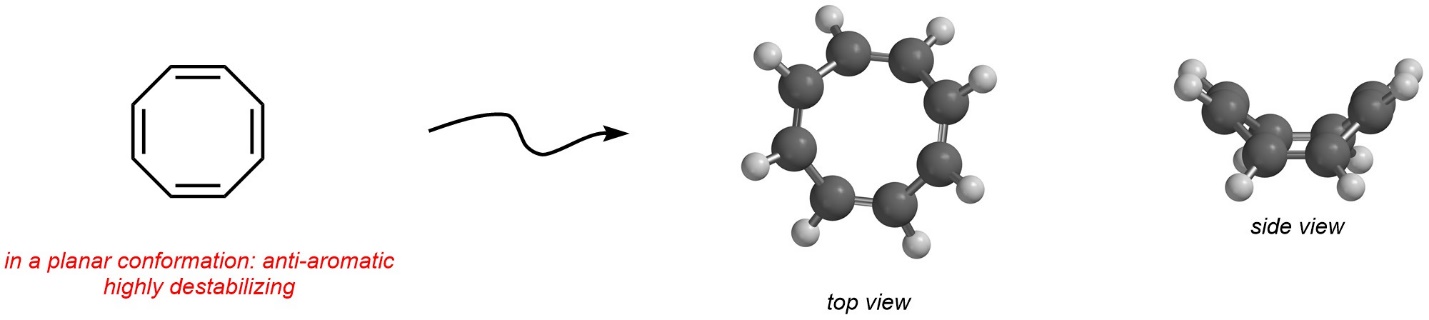
Figure 9.17 – Conformational Change to Avoid Anti-Aromaticity in Cycloocta-1,3,5,7-tetraene.
It is sometimes difficult at an introductory level to recognize when a molecule can adopt a planar conformation to gain aromaticity or can adopt a non-planar conformation to avoid anti-aromaticity. This text will feature only simple examples of these types of molecules.

