9.2. Aromaticity
When more than two p orbitals overlap they are considered conjugated with each other. This is what is being depicted when resonance forms are being drawn and delocalization is being described (see Figure 5.36).
Aromaticity is a special kind of conjugation/delocalization where the p orbitals are in a “loop”, allowing a continuous electron cloud to form a ring above and below the atoms involved (Figure 9.2). This grants special properties to the ring, which are collectively referred to as aromaticity.
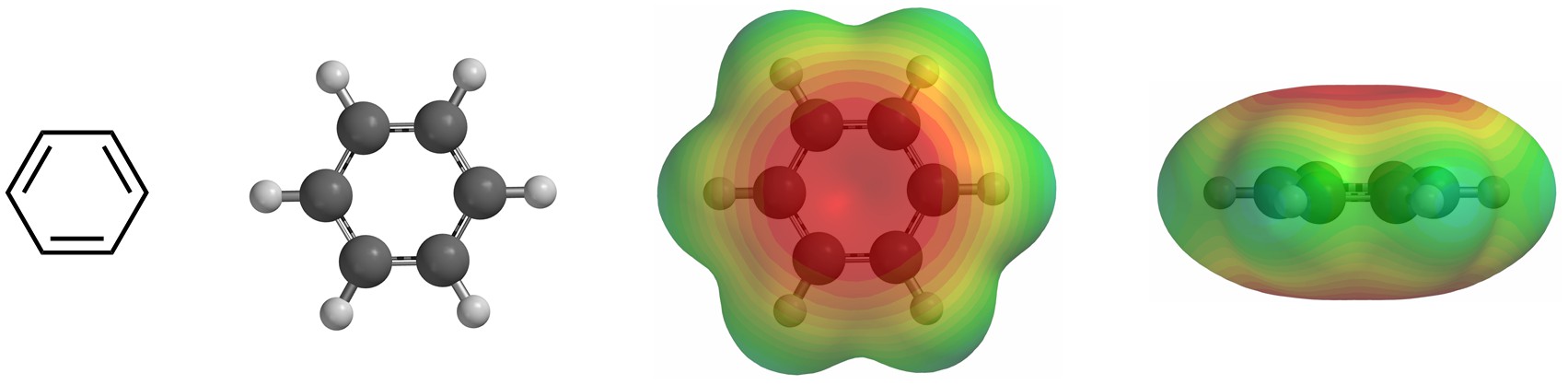
Figure 9.2 – Electrostatic Potential Map of Benzene, an Aromatic Compound.
9.2.1. Criteria for Aromaticity
In order for a molecule (or part of a molecule) to be aromatic it must satisfy four conditions:
- Be cyclic. All aromatic molecules/parts of molecules are (or are made up of) ring systems.
- Have non-orthogonal p orbitals on all atoms in the ring. Every atom in the ring of an aromatic system must have a p orbital, and the p orbitals must all be in the same axis. For example, they must all be aligned pointing up/down (Z-axis) to allow them to overlap with each other.
- Be planar. The aromatic section must be planar/flat to allow the orbitals to overlap and conjugate. Some complex aromatic structures are not perfectly flat, but they are flat enough to allow overlap.
- Obey Hückel’s Rule. Hückel’s rule states that the number of π electrons in the ring system must be a solution to [4n+2], where n is 0 or a positive integer (0, 1, 2, 3, …).
The reasons for Hückel’s rule are beyond the scope of this text (see Section 9.1) but satisfying the rule is vital to obtaining the special properties of aromaticity (see Section 9.2.4.).
9.2.2. Stabilization Relative to Standard Conjugation
Many of the special properties afforded to aromatic rings can be attributed to their high degree of stability. Similar conjugated systems can be compared to aromatic compounds to highlight this difference (Figure 9.3).
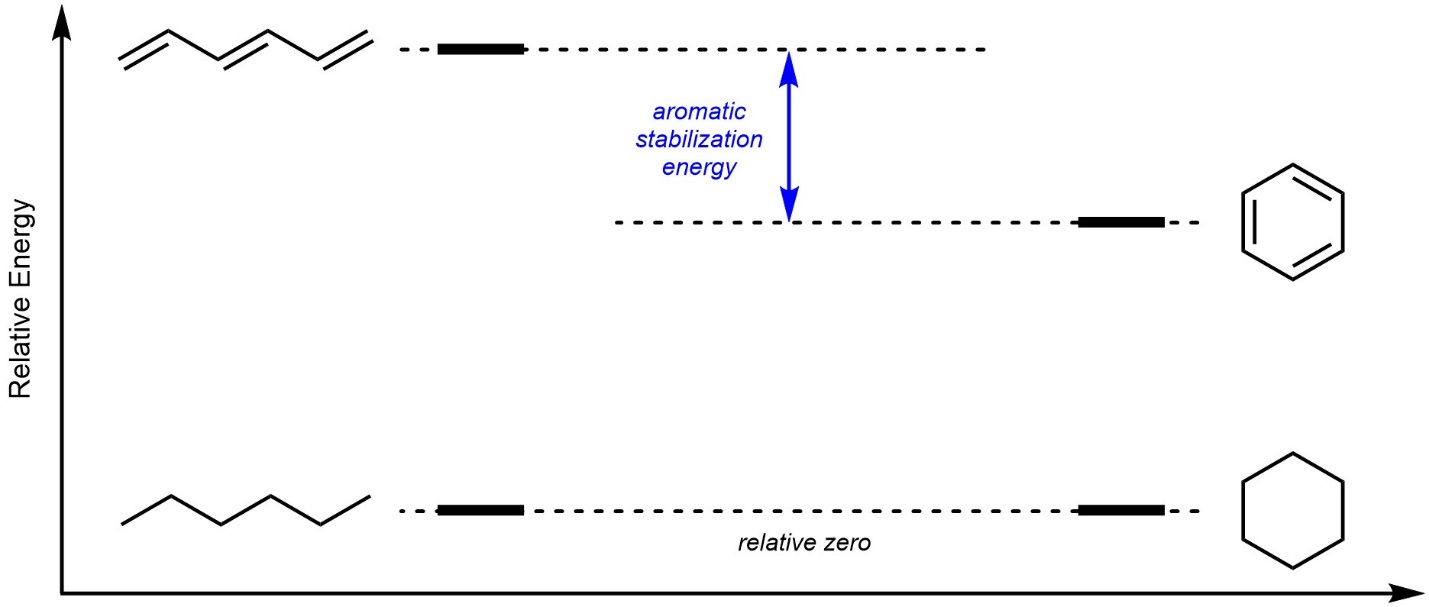
Figure 9.3 – Aromatic Stabilization Energy of Benzene.
Recall that most π bonds are higher in energy (less stable) than σ bonds. This is true even when conjugation/delocalization is present. For example, relative to hexane, the conjugated molecule 1,3,5-hexatriene (either the E or Z isomer) is higher in energy. Despite the apparent similarities, the aromatic compound benzene is significantly less destabilized (i.e. less high in energy relative to the equivalent molecule without π bonds). The unexpected energy that is gained by being aromatic is referred to as aromatic stabilization energy.
Aromatic stabilization dramatically affects the chemical reactivity of compounds. Reactions that lead to an aromatic ring from a non-aromatic starting material are substantially faster than they otherwise would be. More importantly, reactions that remove aromaticity from a system are heavily disfavoured. For example, the addition of Br2 across an alkene is favourable (Scheme 9.1). The addition of Br2 across a conjugated alkene is slightly slower but still favourable. However, the addition of Br2 across an “alkene” in an aromatic ring does not proceed. Even in the presence of a catalyst, a different reaction instead occurs which does not disrupt aromaticity (see Section 10).
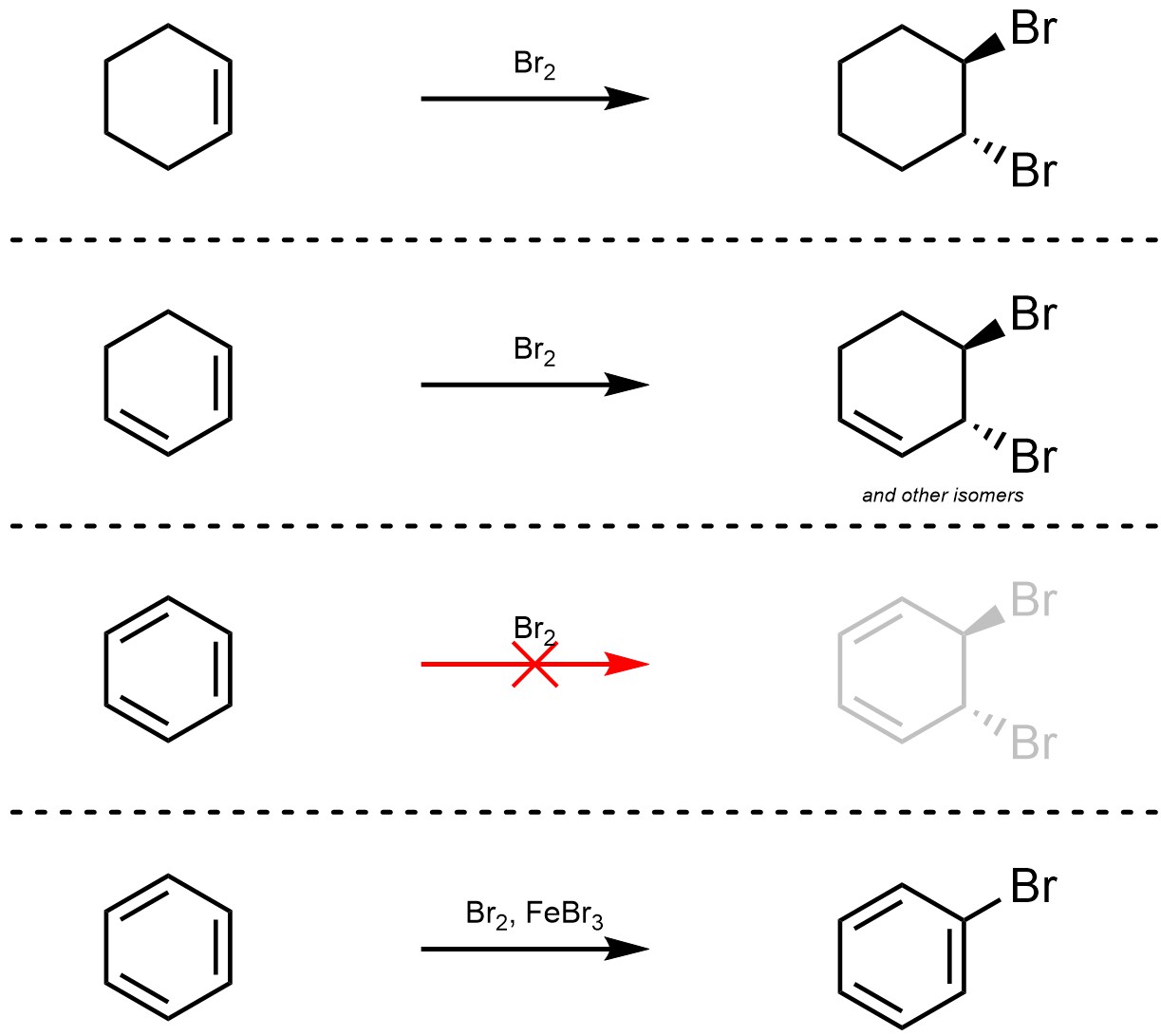
Scheme 9.1 – Comparison of Reactivity of Alkenes and Aromatic Rings.
9.2.3. Aromaticity and Resonance
All aromatic structures can be represented by at least two resonance forms (Figure 9.4).
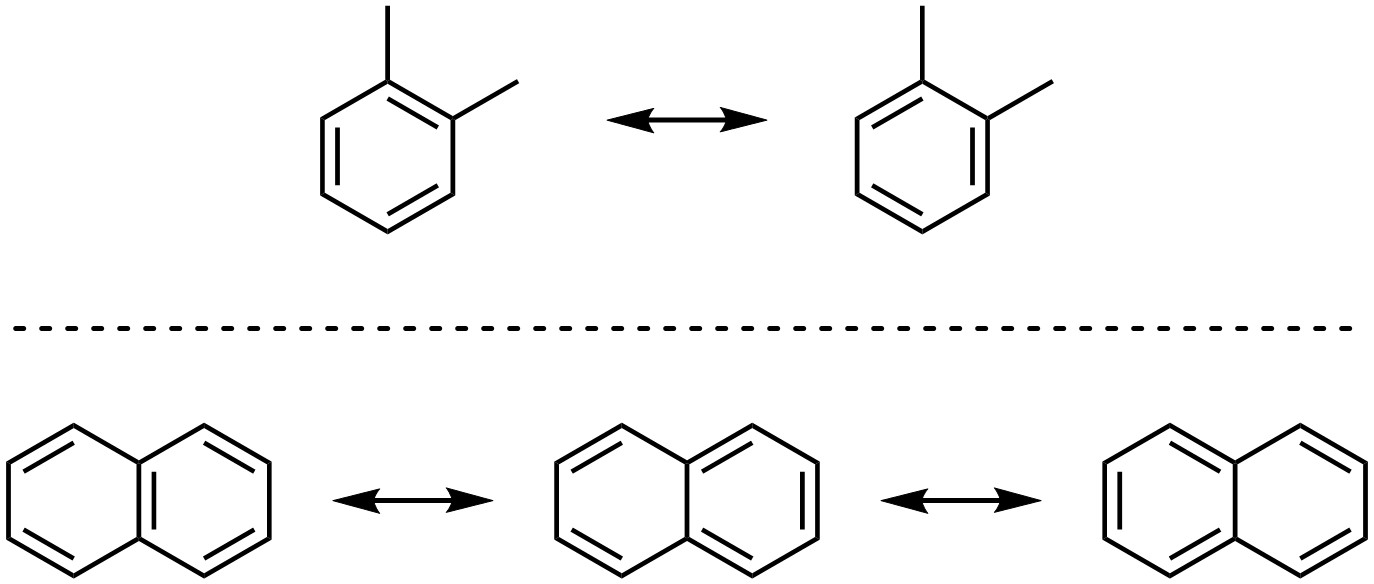
Figure 9.4 – Resonance Structures of 1,2-Dimethylbenzene and Naphthalene.
To highlight the delocalization some sources use dotted lines, and very old sources may indicate aromaticity using a circle within the ring in place of π bonds (see Section 5.5; Figure 9.5). The use of either of these conventions is heavily discouraged as these depictions obscure the details of reaction mechanisms with aromatic rings.
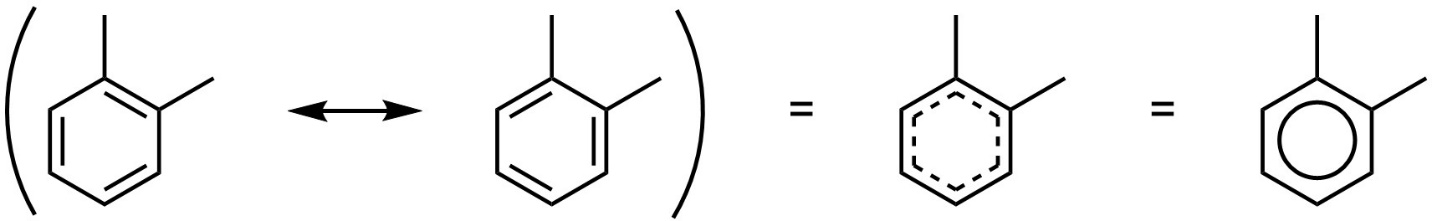
Figure 9.5 – Alternate Representations of Aromaticity.
9.2.4. Anti-Aromaticity and Hückel’s Rule
Satisfying Hückel’s Rule is vital for aromaticity. Having [4n+2] electrons in the π system of the ring makes the ring aromatic and grants aromatic stabilization. However, if a molecule satisfies the first three criteria for aromaticity and instead has [4n] π electrons in the ring system it becomes anti-aromatic and is heavily destabilized.
A simple example of this effect is the stability of benzene compared to cyclobutadiene (Figure 9.6). Benzene satisfies the first three conditions and has 6 π electrons in the ring system [6 = 4(1)+2]. It is therefore aromatic. In a jar on a shelf, it is stable for decades. Cyclobutadiene satisfies the first three conditions and has 4 π electrons in the ring system [4 = 4(1)]. It is therefore anti-aromatic. In a jar on a shelf, it is stable for less than half of a second.
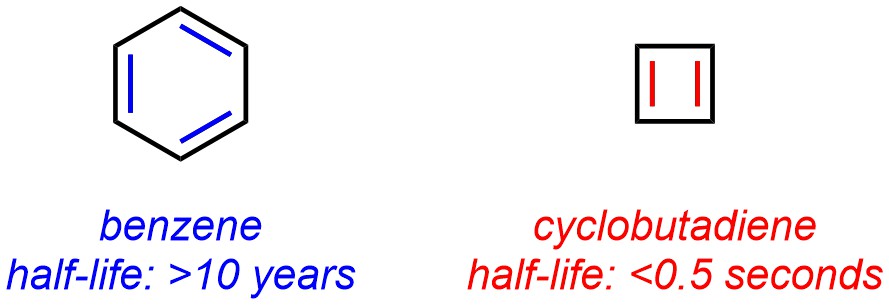
Figure 9.6 – Comparison of Half-Lives for Aromatic Benzene and Anti-Aromatic Cyclobutadiene.

