11.2. Substitution Reactions: SN2 Reactions
Nucleophilic substitution reactions (sometimes called Displacement reactions) can occur with one or two steps in the mechanism. With one step in the mechanism, SN2 reactions are functionally identical to acid-base reactions. The main difference from their counterpart is that they are stereospecific; the stereochemistry of the product will be dictated by the reaction.
11.2.1. Mechanism
Nucleophilic substitution reactions that follow the SN2 mechanism have one step (Scheme 11.2). The nucleophile attacks the electrophile, creating a new bond. This occurs at the same time that the bond between the electrophile and the leaving group breaks. The new bond forms at the same time that the old bond breaks.
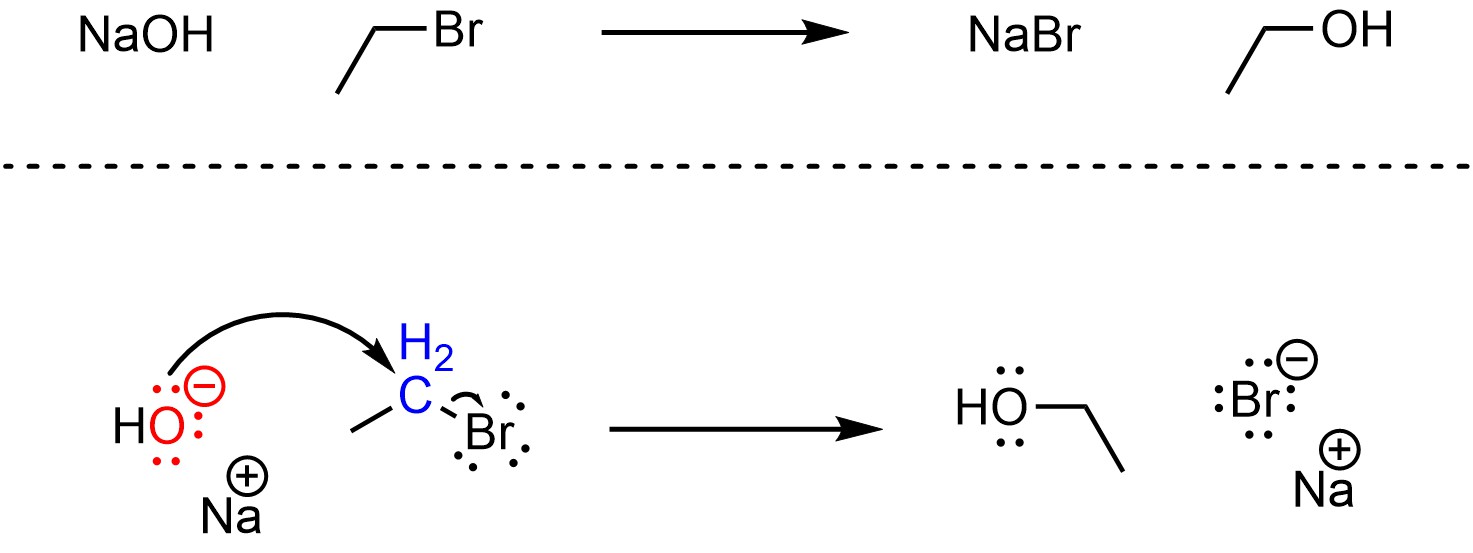
Scheme 11.2 – Reaction Mechanism for SN2 Nucleophilic Substitution of Bromoethane with Sodium Hydroxide.
Depending on the specific reaction that is occurring there may be additional steps before or after the substitution step. For example, the nucleophile or electrophile may be activated prior to the substitution, or there may be a protonation or deprotonation after the nucleophile attacks the electrophile (Scheme 11.3). These extra steps do not change the classification of the mechanism for the substitution step; if the old bond breaks at the same time the new bond forms, it is still classed as an SN2 nucleophilic substitution reaction.
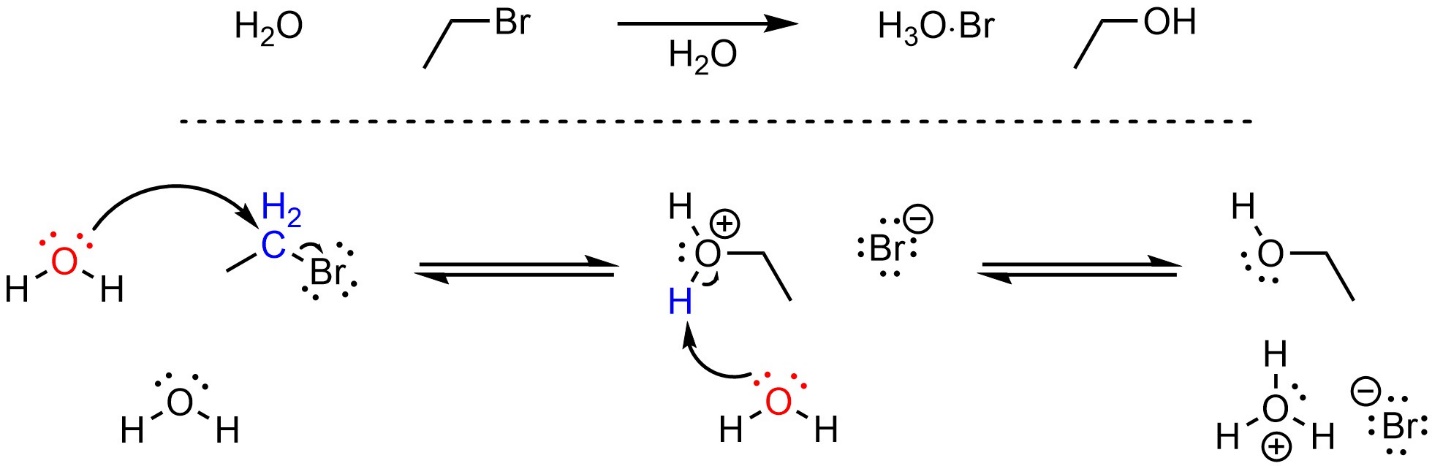
Scheme 11.3 – Reaction Mechanism for SN2 Nucleophilic Substitution of Bromoethane with Water.
Other examples of these types of SN2 reactions include acid- or base-catalyzed openings of epoxides (see Sections 8.10.1 and 8.10.2), which follow an SN2 mechanism for the ring-opening but have additional steps before and after the nucleophilic substitution step.
11.2.2. Reaction Coordinate and Rate-Determining Step
There is only one step in an SN2 substitution reaction. By definition, it is the rate-determining step (Figure 11.1). The overall reaction is slightly exergonic.
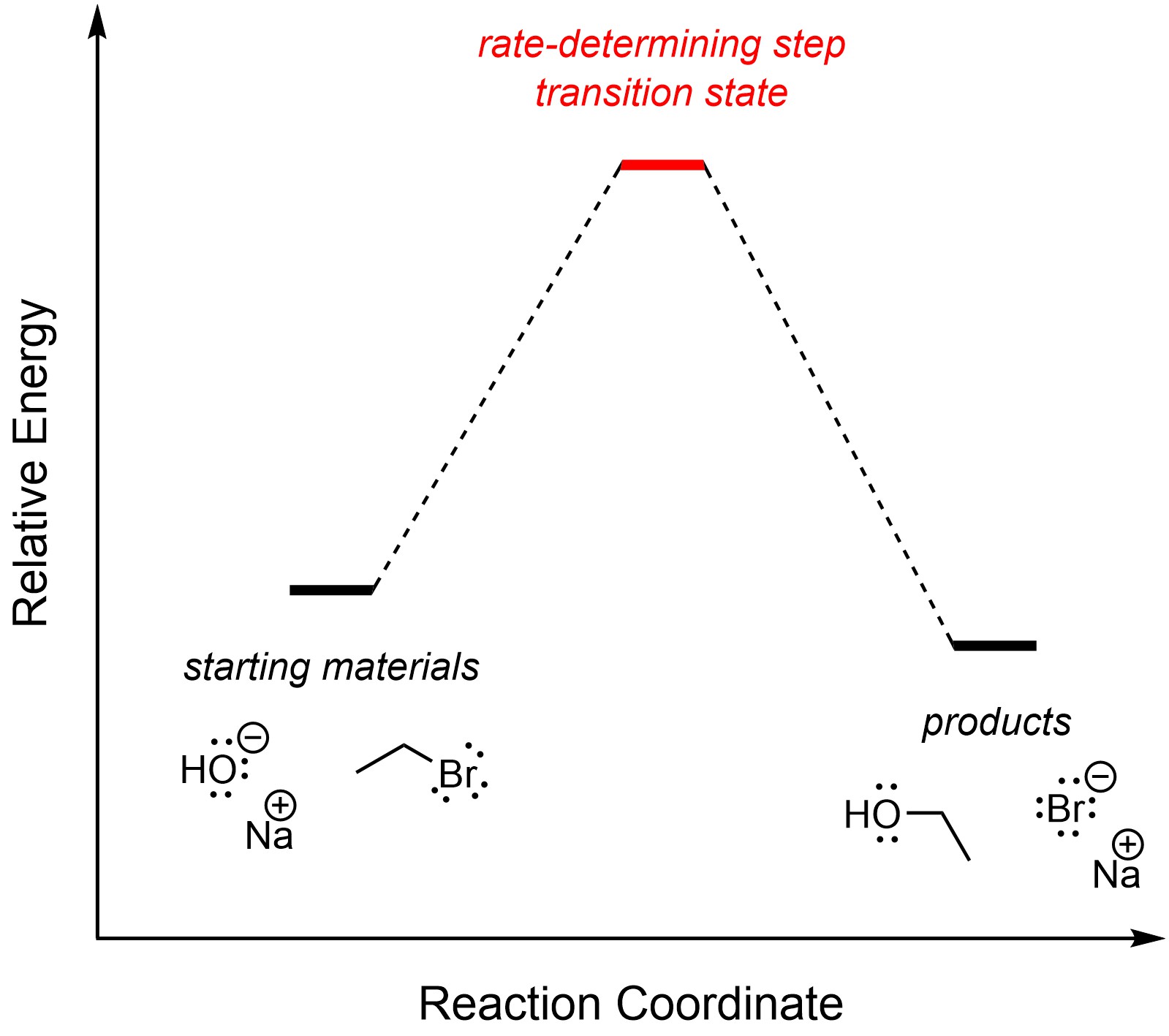
Figure 11.1 – Reaction Coordinate for SN2 Nucleophilic Substitution of Bromoethane with Sodium hydroxide.
11.2.3. Stereoselectivity – Stereospecific: Inversion
Recall that when two orbitals overlap constructively they form a covalent bond (σ or π; see Section 1.3.1). However, when they overlap deconstructively they form an anti-bonding orbital (σ* or π*; see Section 1.3.1). Usually, in order to break a covalent bond electron density must be put into its corresponding anti-bonding orbital (Figure 11.2). This also makes a new bond between the atom that donated the electrons and the atom that was attacked.
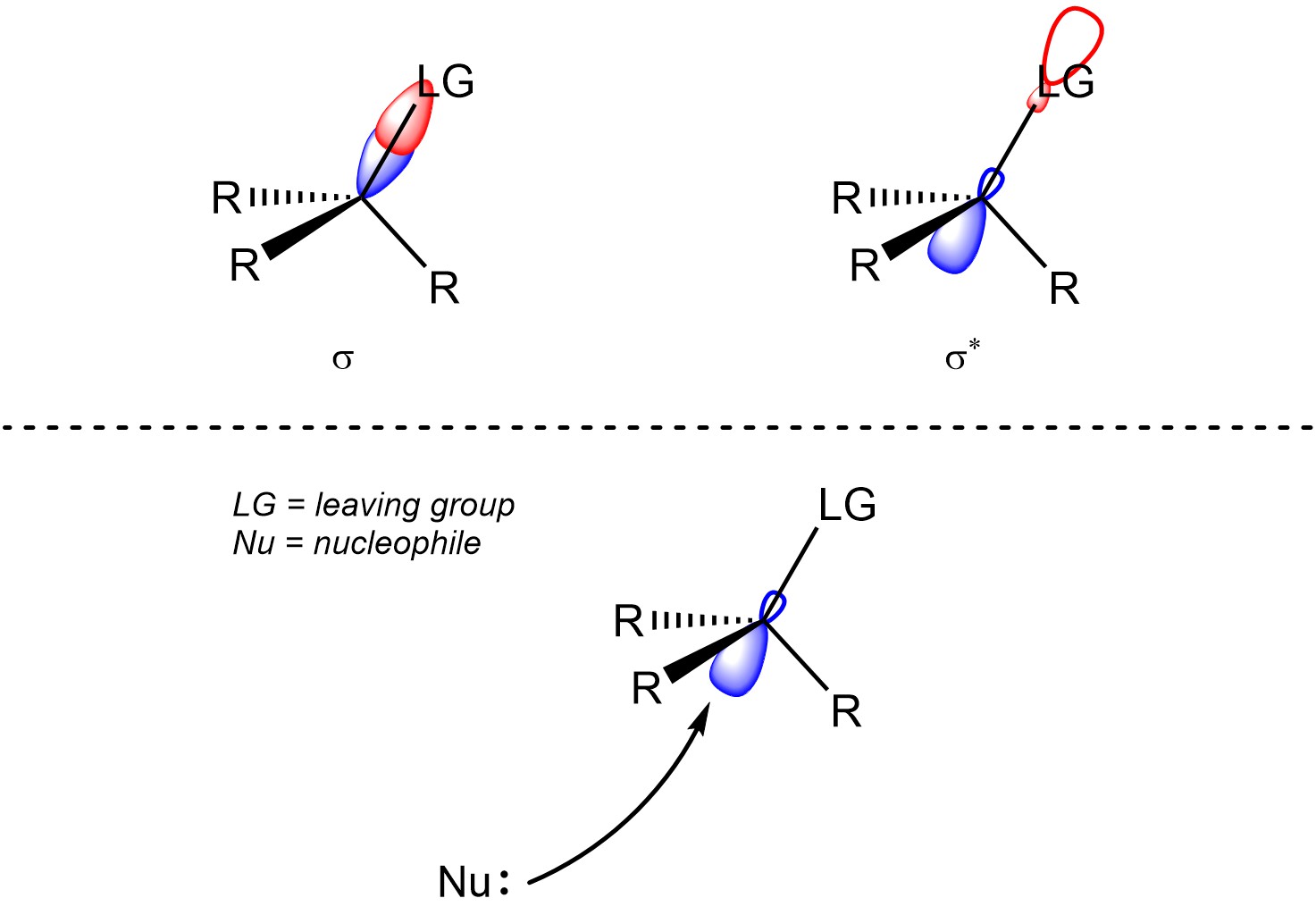
Figure 11.2 – A σ Bond and the Corresponding σ* Anti-Bonding Orbital; Representation of the Geometric Constraint of Nucleophilic Attacks into the Corresponding σ* Anti-Bonding Orbital for SN2 Reactions.
It is only possible for the new bond to form at the same time that the old bond is broken by attacking from the side opposite to the leaving group (Scheme 11.4). As a result, the new group will be attached from the opposite side as the old group. This is commonly referred to as inversion of configuration; R stereocentres become (different) S stereocentres, S stereocentres become (different) R stereocentres. Alternatively, if the starting material and product are drawn in the same conformation and from the same perspective then the new bond will be wedged if the old bond was hashed (and vice versa).
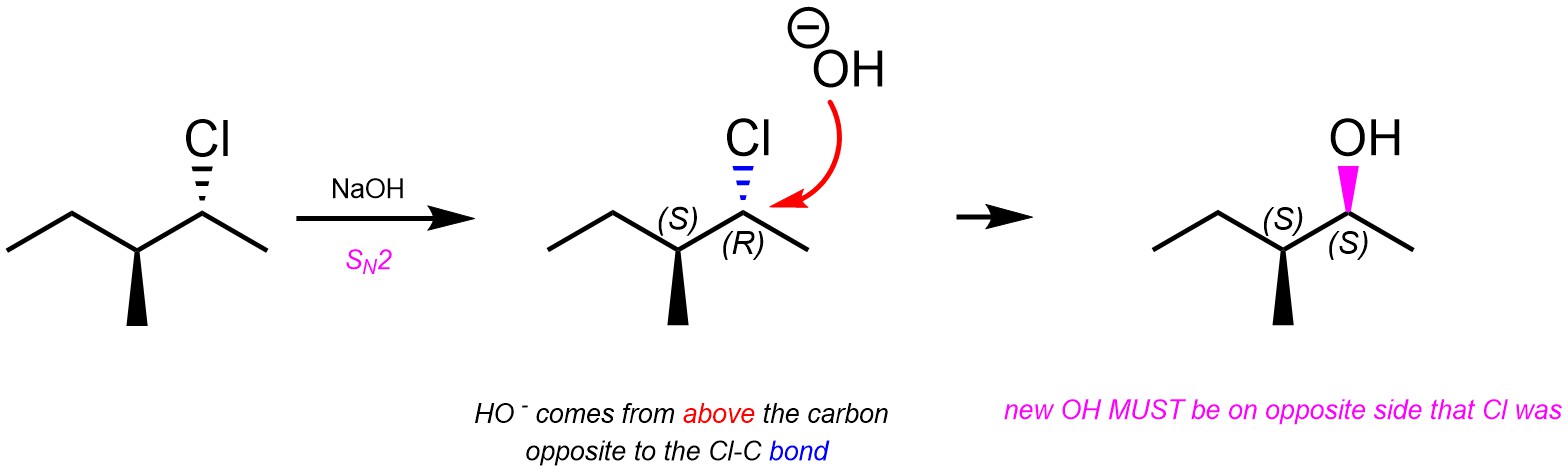
Scheme 11.4 – Stereospecific SN2 Nucleophilic Substitution of Chlorine in (2R,3S)-2-Chloro-3-methylpentane Using Sodium Hydroxide.
Because the stereoselectivity comes from the mechanism, this step is stereospecific. It does not select for a specific enantiomer nor a specific diastereomer, it only requires that the stereocentre that was attacked be inverted.
11.2.4. Reaction Rate
Nucleophilic substitution reactions that follow SN2 pathways have only one step in their mechanisms. The “2” in the name refers not to the mechanism but to the reaction’s rate law, which is second-order and dependent on two things: the nucleophile and the electrophile. This is because both of these compounds are involved in the rate-determining step.
Because the reaction rate is affected by the nucleophile, anything that makes the nucleophile more nucleophilic will increase the rate of the reaction. Because the reaction rate is affected by the electrophile, anything that makes the electrophile more electrophilic will increase the rate of the reaction. The opposite is also true; weaker nucleophiles/electrophiles will make the reaction slower.
11.2.4.1. Effects from the Nucleophile
The rate of the reaction is affected by the strength of the nucleophile. All factors that affected basicity/nucleophilicity (see Section 6.3.) still apply.
Anionic compounds are more nucleophilic/basic than neutral equivalents (Scheme 11.5).
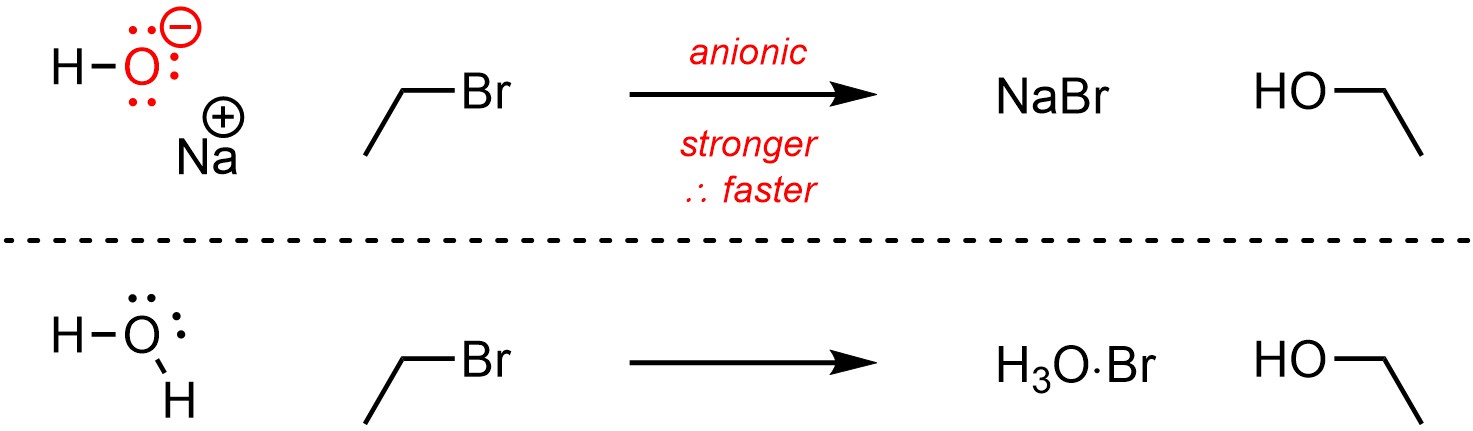
Scheme 11.5 – Example of Effect of Charge on Nucleophilicity.
Less electronegative atoms are more nucleophilic/basic than more electronegative atoms (Scheme 11.6).
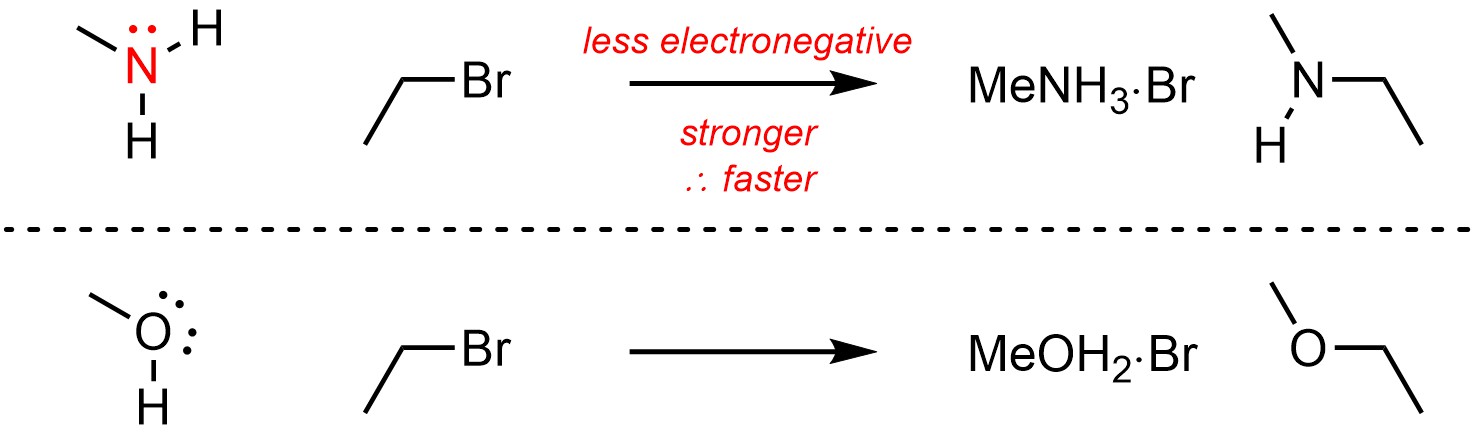
Scheme 11.6 – Example of Effect of Electronegativity on Nucleophilicity.
Having multiple resonance forms makes compounds more stable. The more stable a compound is, the less nucleophilic it is (Scheme 11.7).
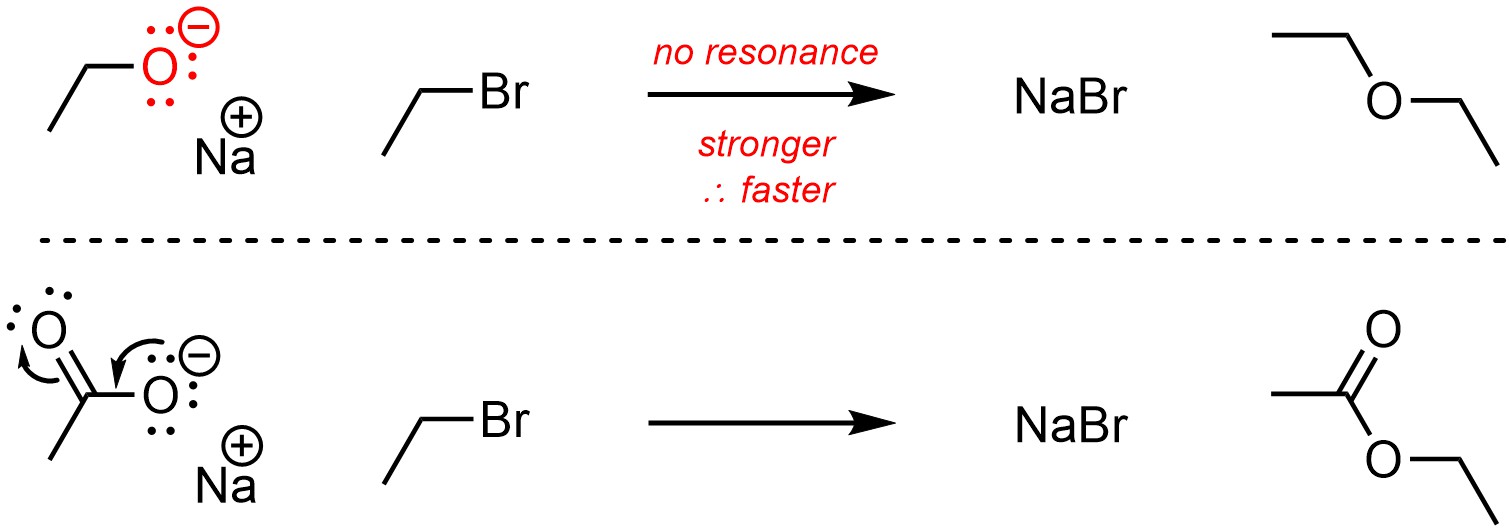
Scheme 11.7 – Example of Effect of Resonance on Nucleophilicity.
Unlike with acid-base reactions, steric strain does affect how nucleophilic an atom is. Steric interactions from large (“bulky”) groups near a nucleophile will make it a less effective nucleophile by making it less accessible (Scheme 11.8).
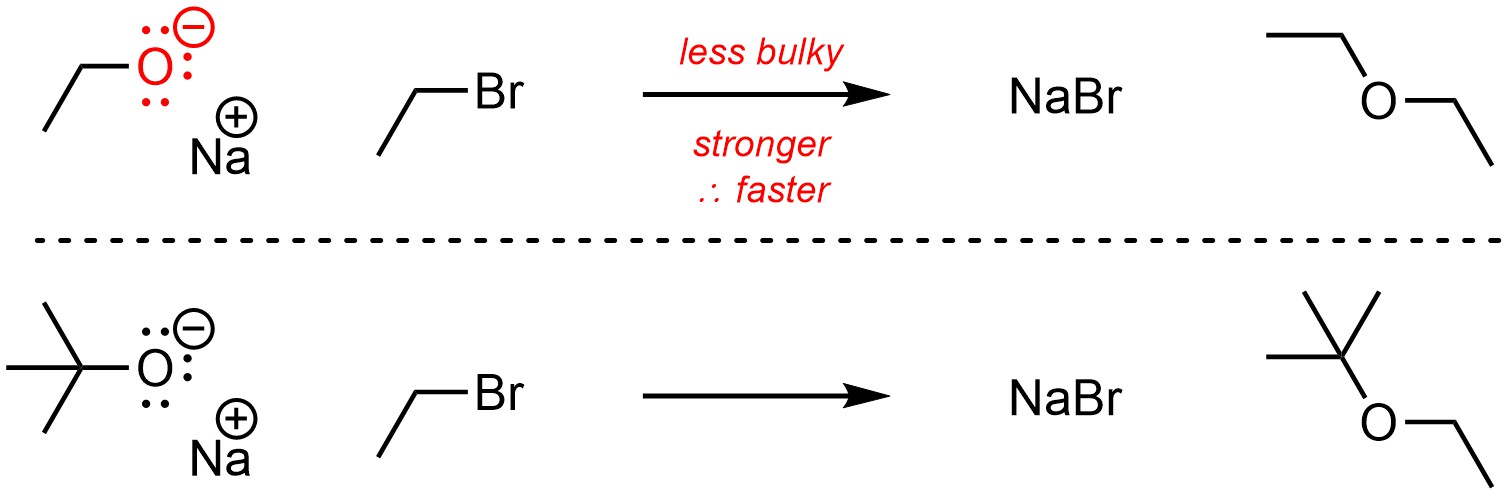
Scheme 11.8 – Example of Effect of Steric Strain (Bulk) on Nucleophilicity.
11.2.4.2. Effects from the Electrophile
The rate of the reaction is affected by the strength of the electrophile. All factors that affected leaving group ability (see Section 7.7.) still apply; generally, the conjugate bases of strong acids are very good leaving groups, the conjugate bases of moderate acids are good to moderate leaving groups, and the conjugate bases of weaker acids are moderate to poor leaving groups. For example, water (pKa = 15.7) and ethanol (pKa = 15.9) are weak acids so their conjugate bases (hydroxide and ethoxide) are poor leaving groups.
Cationic groups are better leaving groups than neutral equivalents (Scheme 11.9).
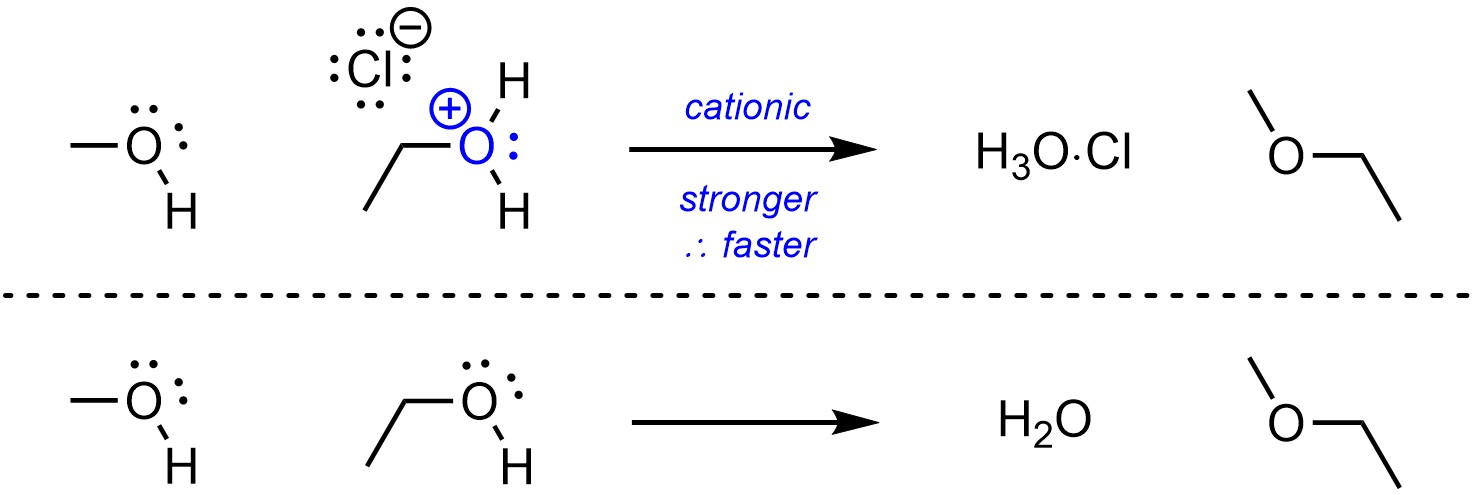
Scheme 11.9 – Example of Effect of Charge on Electrophilicity.
More electronegative atoms are better leaving groups than less electronegative atoms (Scheme 11.10).
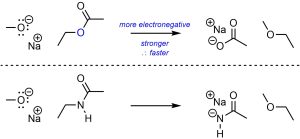
Scheme 11.10 – Example of Effect of Electronegativity on Electrophilicity.
Atoms that have access to much larger orbitals (are in lower rows on the Periodic Table) are better leaving groups than those with access to smaller orbitals (Scheme 11.11). This is more important than electronegativity.
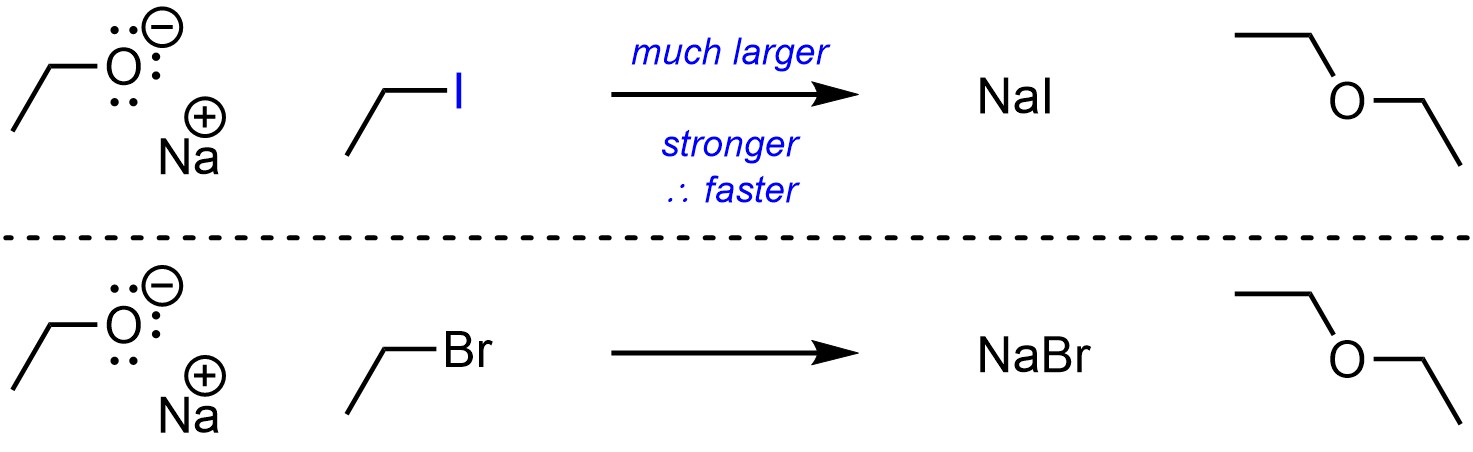
Scheme 11.11 – Example of Effect of Atomic Size on Electrophilicity.
Having multiple resonance forms makes compounds more stable. The more stable a compound is, the better a leaving group it is (Scheme 11.12).
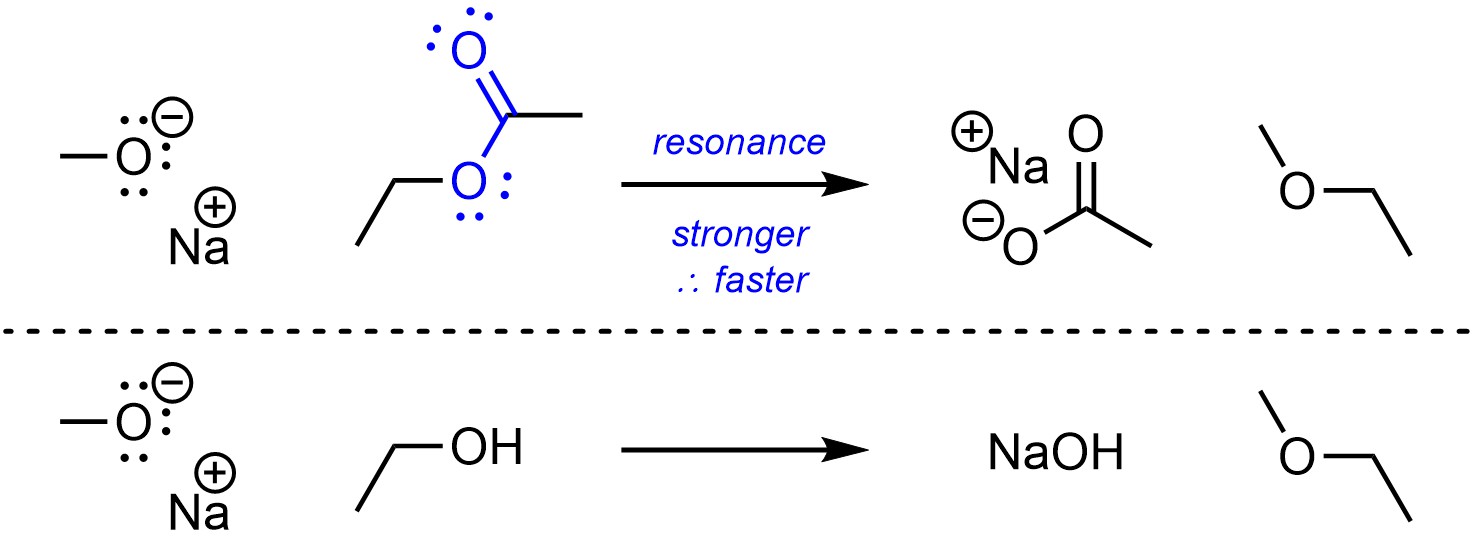
Scheme 11.12 – Example of Effect of Resonance on Electrophilicity.
Unlike with acid-base reactions, steric strain does affect how electrophilic an atom is. Steric interactions from large (“bulky”) groups near an electrophile will make it a less effective electrophile by making it less accessible (Scheme 11.13).
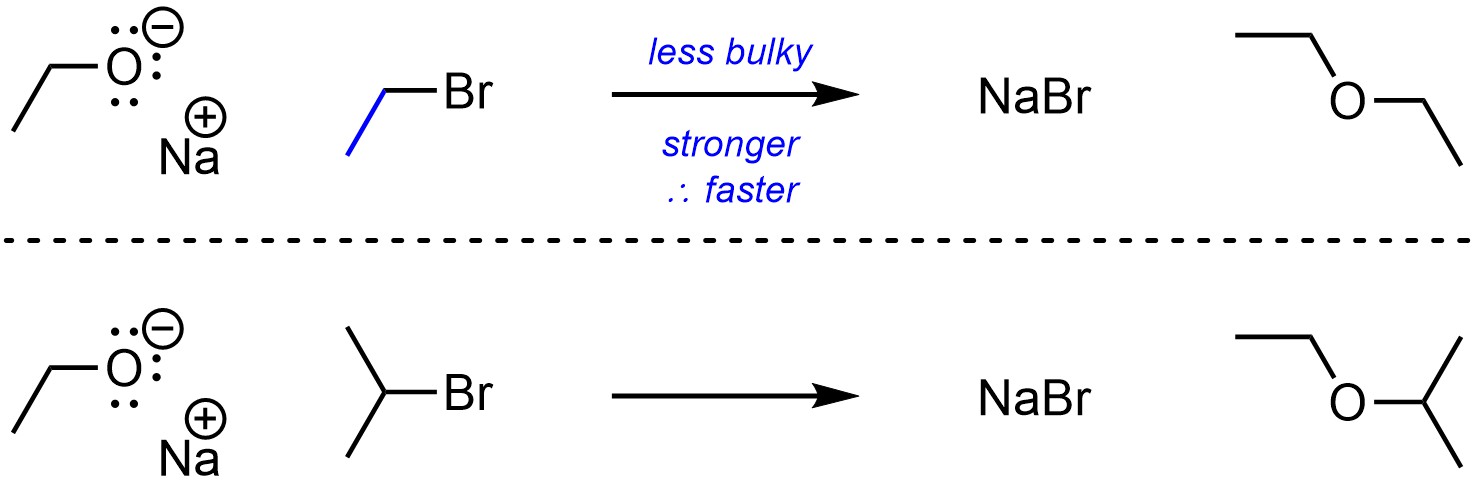
Scheme 11.13 – Example of Effect of Steric Strain (Bulk) on Electrophilicity.

