8.1. (Very) Brief Refresher of the Basics
Recall that atoms which are sp2 hybridized have trigonal planar geometries. This means that they occupy the same plane as the atom(s) they are directly bonded to. The highlighted atoms below are all in exactly the same plane regardless of conformation (Figure 8.1).
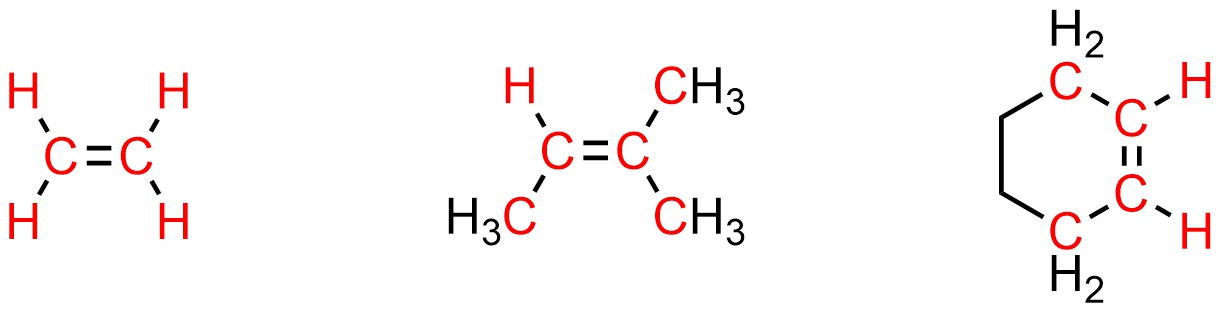
Figure 8.1 – Highlighted Examples Showing Planarity of Alkenes.
The p orbitals used to make the π bonds in these alkenes are orthogonal to the plane of the atoms; the electron density of the π bond is located directly above and below the plane of the alkene (Figure 8.2).
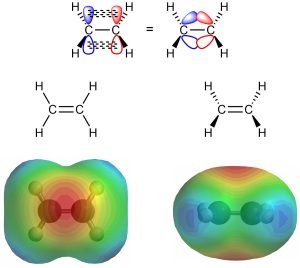
Figure 8.2 – Electron Density in Ethylene.
Most π bonds are higher in energy (less stable) than σ bonds. As a result, they are generally weaker and relatively easy to break. With polar π bonds this results in being electrophilic at whichever atom of the π bond has less electron density (see Chapter 7). With non-polar (or weakly polar) π bonds the opposite occurs. Because the π bond represents an area of high electron density localized in a bond that is relatively easy to break, the electron density can be used to attack an electrophile. Non-polar π bonds are nucleophiles.

