8.11. Reaction: Addition of H-H
It is possible to add a hydrogen (H) and another hydrogen (H) across an alkene (Scheme 8.36). This is often referred to as hydrogenation. This requires the use of a transition metal catalyst, most commonly with platinum or palladium.
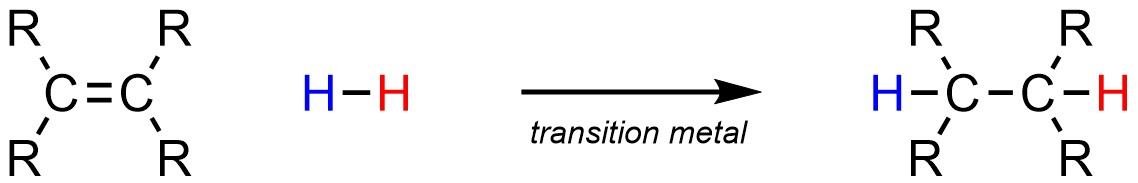
Scheme 8.36 – Generalized Reaction Equation for Addition of H-H Across an Alkene.
8.11.1. “Mechanism”
The most commonly used catalysts for this reaction (Pd(C) [palladium on carbon] and Lindlar’s Catalyst) are organometallic compounds. Recall that the reactivity and properties of organometallics are complex, and most institutions have one or more entire courses dedicated to their study. As a result, an in-depth discussion of the mechanisms for these reactions is beyond the scope of this text. However, they can be visualized by approximating the metallic component as a metal surface (Figure 8.9). At an introductory level a detailed understanding of this mechanism is not required, it is provided only to supplement understanding.
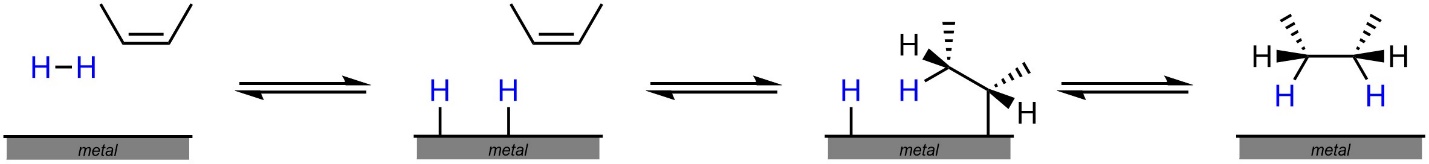
Figure 8.9 – Hydrogenation of an Alkene, Details Omitted.
8.11.2. Regioselectivity
Because both groups being added are identical, there are no regioisomers and regioselectivity is not a concern.
8.11.3. Stereoselectivity – Stereospecific: cis
As a result of the mechanism the two hydrogens must be added cis relative to each other (cis addition, Scheme 8.37).
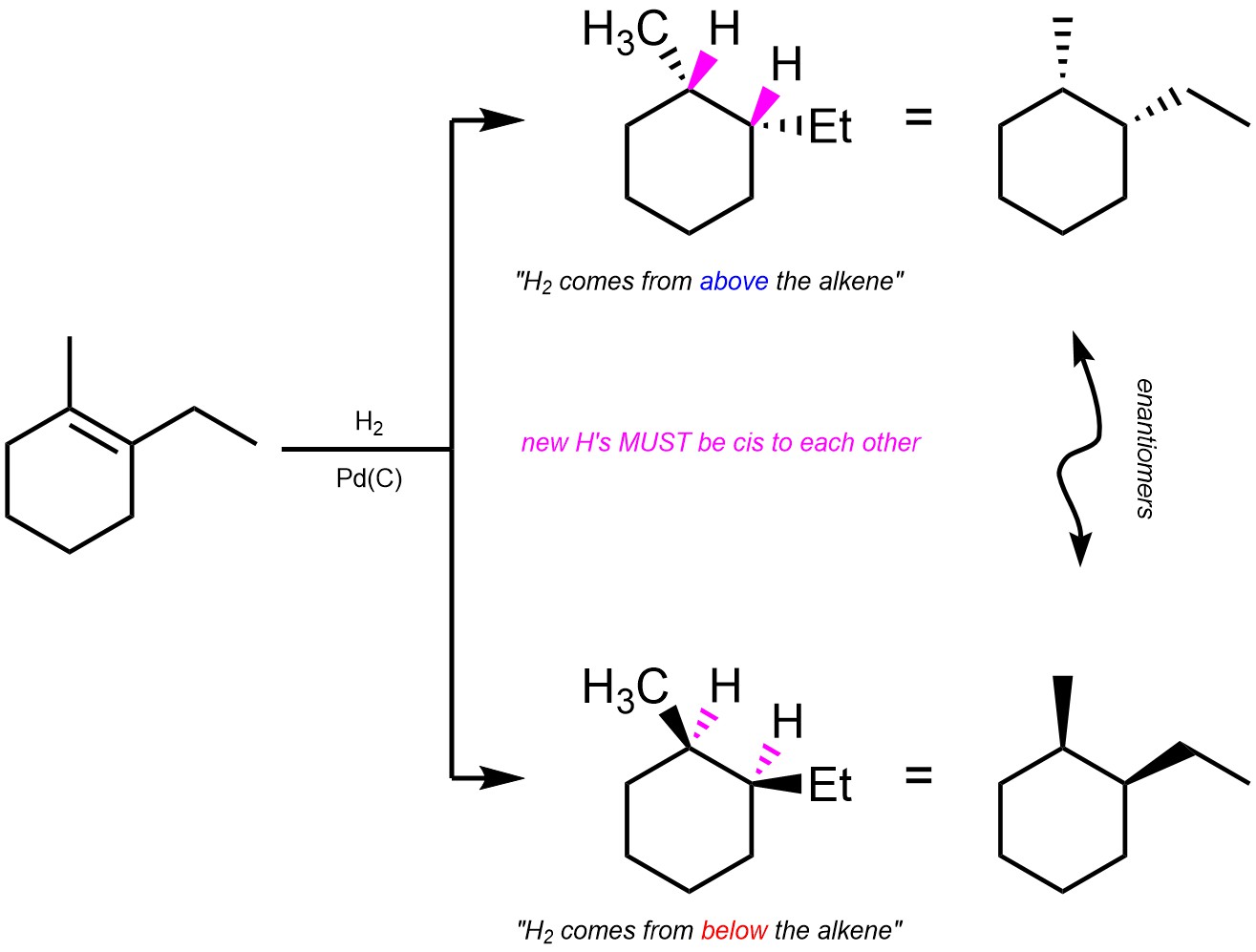
Scheme 8.37 – Diastereospecific Addition of Cl-OR Across the Alkene of Methylcyclohexene.
It is very important to remember that this is a form of diastereoselectivity (by being diastereospecific). There is no enantioselectivity.
8.11.4. Alkenes vs. Alkynes and Lindlar’s Catalyst
Most catalysts, including the most common one (Pd(C); palladium on carbon), will hydrogenate both alkenes and alkynes down to alkanes (adding one or two equivalents of H2 respectively). However, Lindlar’s Catalyst can only hydrogenate alkynes (Scheme 8.38). Lindlar’s catalyst is a mixture of several compounds. The specific details of what comprises Lindlar’s catalyst, its mechanism of hydrogenation, and why it cannot add hydrogen across alkenes are well beyond the scope of this text. It is important only to understand that using Lindlar’s Catalyst will only hydrogenate alkynes, and that it will still do so in a stereospecific (diastereospecific, cis addition) way.
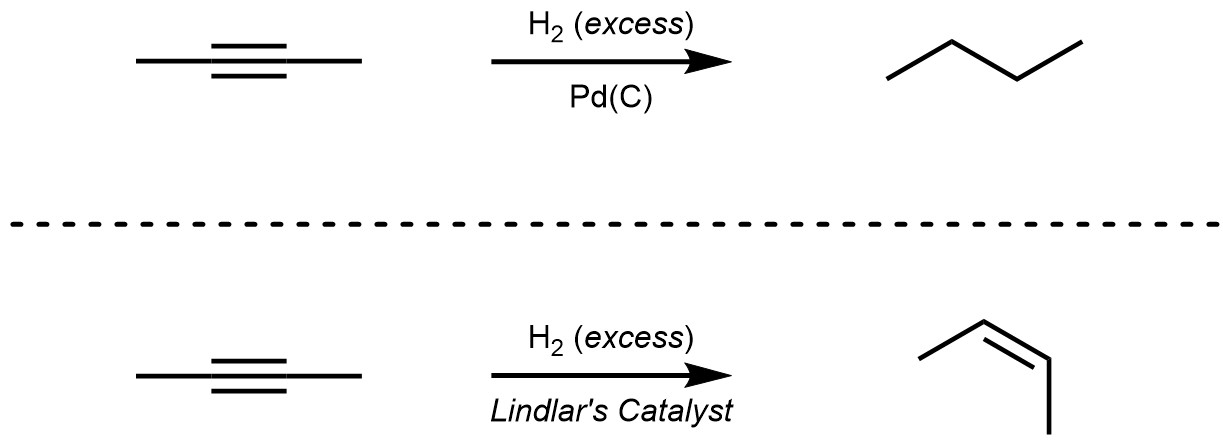
Scheme 8.38 – Comparison of Pd(C) and Lindlar’s Catalyst for Hydrogenation of But-2-yne.

