13.4. Reaction Rates and Relative Reactivity
Because the rate-determining step involves the nucleophile and electrophile the relative strength of each can affect the rate of the reaction.
All factors that affect nucleophilicity (see Sections 7.2.2 and 11.2.5.1) still apply; whichever compound is a stronger base is also a stronger nucleophile but steric strain from large (“bulky”) groups near a nucleophile will make it less nucleophilic.
Typically, the strength of the electrophile varies more and has a larger effect on the rate of addition-elimination reactions. By far the easiest way to compare these functional groups for electrophilicity is to evaluate the leaving group as an electron-donating or electron-withdrawing group (see Sections 10.10.1-10.10.5; Figure 13.2).
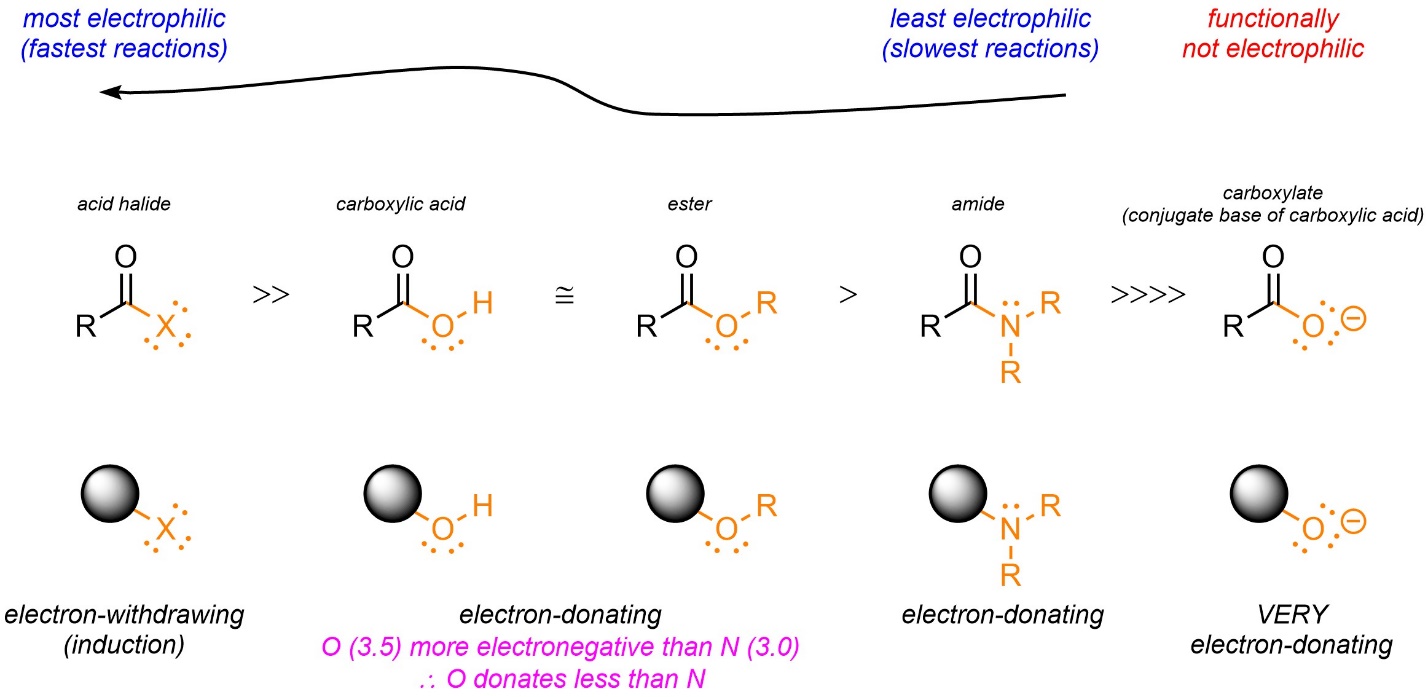
Figure 13.2 – Qualitative Comparison of Electrophilicity of Carbonyl-Containing Functional Groups by Evaluation of the Leaving Group as Electron-Withdrawing or Electron-Donating.
Electron-withdrawing groups such as halides (as part of an acid halide) will undergo addition-elimination reactions very quickly. For electron-donating groups the stronger the electron-donation, the slower the reaction. For example, the nitrogen of an amide is a stronger electron-donating group than the oxygen of an ester. Addition-elimination reactions with esters are (much) faster than with amides. Very strong electron-donation, such as from an anionic group, may lower the electrophilicity so much that addition-elimination reactions are not possible.
It is possible to compare and rank the relative electrophilicities of any appropriate functional groups for addition-elimination reactions, including uncommon ones. For example, an anhydride (a functional group not covered in Section 2.2) can be evaluated as being less electrophilic than an acid halide but more electrophilic than a carboxylic acid/ester by this method (Figure 13.3).
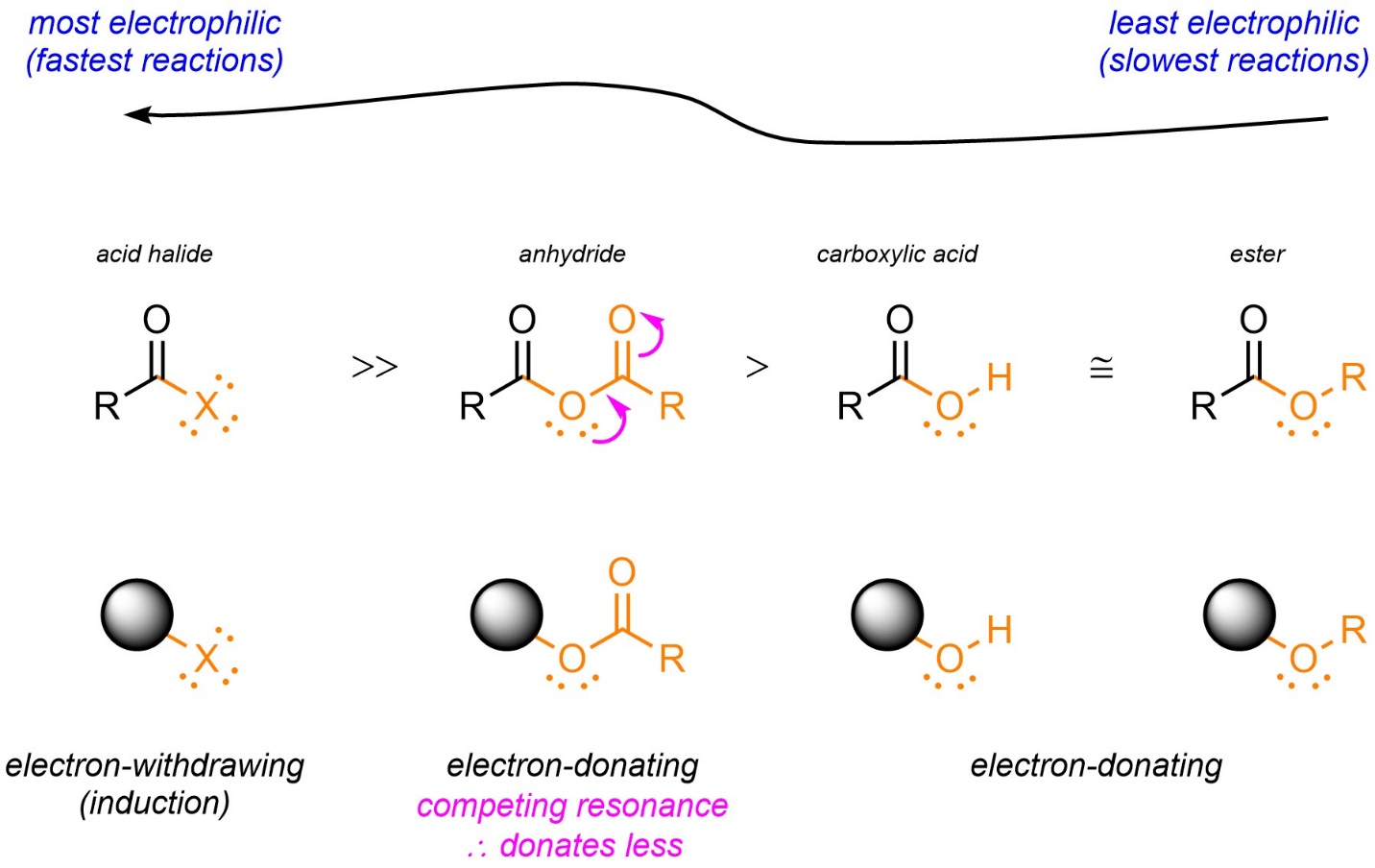
Figure 13.3 – Qualitative Comparison of Electrophilicities of Acid Halides, Anhydrides, and Carboxylic Acids/Esters by Evaluation of the Leaving Group as Electron-Withdrawing or Electron-Donating.
Many texts suggest an alternative approach to compare electrophilicities: evaluation of resonance contributions. Consider the resonance forms of an acid chloride, an ester, and a carboxylate (Figure 13.4). For all three the left resonance form contributes the most.
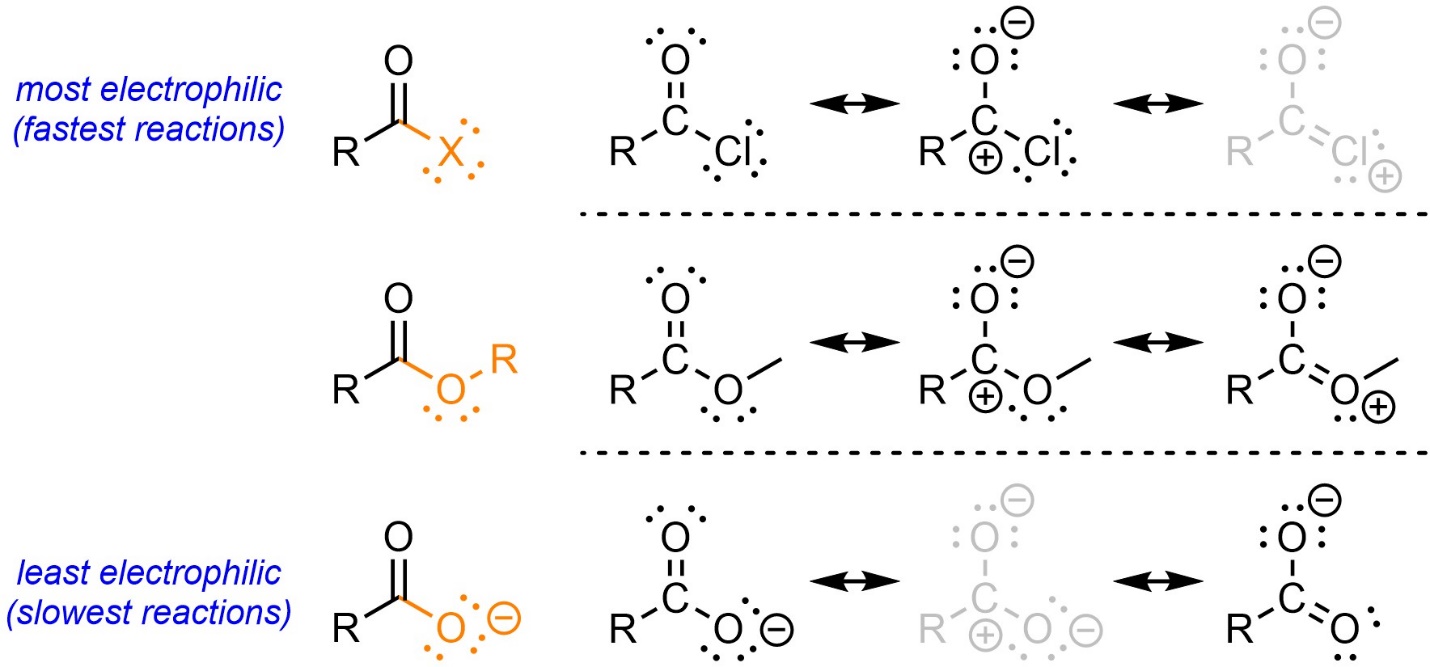
Figure 13.4 – Qualitative Comparison of Electrophilicity of Carbonyl-Containing Functional Groups by Evaluation of Resonance Forms.
For the acid chloride the right resonance form functionally does not exist/contribute. Relative to the other two functional groups its carbon has more cationic character; it must be the most electrophilic. For the carboxylate the middle resonance form functionally does not exist/contribute. Relative to the other two functional groups its carbon has (much) less cationic character; it must be the least electrophilic. This can be applied to other functional groups.
Use of either method to compare and rank electrophilicity is acceptable.

