7.1. (Very) Brief Refresher of the Basics
Recall that a carbon double bonded to an oxygen (with two other groups on the carbon) is present in many functional groups (see Figure 2.25). This arrangement of atoms is so common that it has a special name: a carbonyl. Carbonyls are not functional groups on their own. However, they are part of many functional groups. Because this element is common to so many groups, it imparts a similar type of reactivity to all groups with it.
Oxygen is more electronegative than carbon, so all carbonyl-containing functional groups have permanent dipoles (Figure 7.1). This becomes even more apparent when the resonance forms of carbonyls are considered. It is important to remember that depending on what the two other groups are, additional resonance forms may be possible. Based on these it should be clear that there is a permanent excess of electron density on the oxygen and a permanent deficiency of electron density on the carbon.
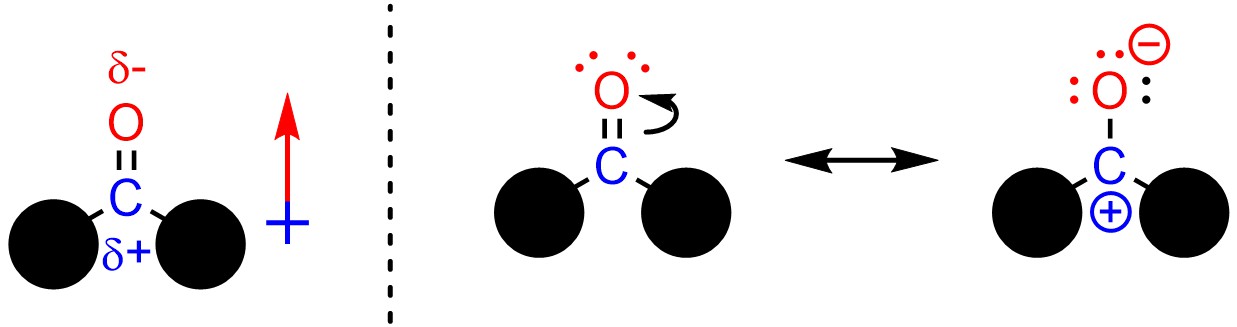
Figure 7.1 – Dipole and Resonance Forms of a Generic Carbonyl Group.

