Chapter 1 Practice Problems
Answers for these practice problems are on the next page.
A good approach is to answer all of the questions on a piece of paper and then check your answers. This avoids accidentally seeing the answer(s) for questions you have not done yet.
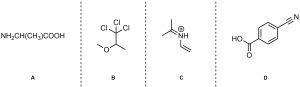
Q1.1: How many sigma (σ) and pi (π) bonds are there in these molecules?
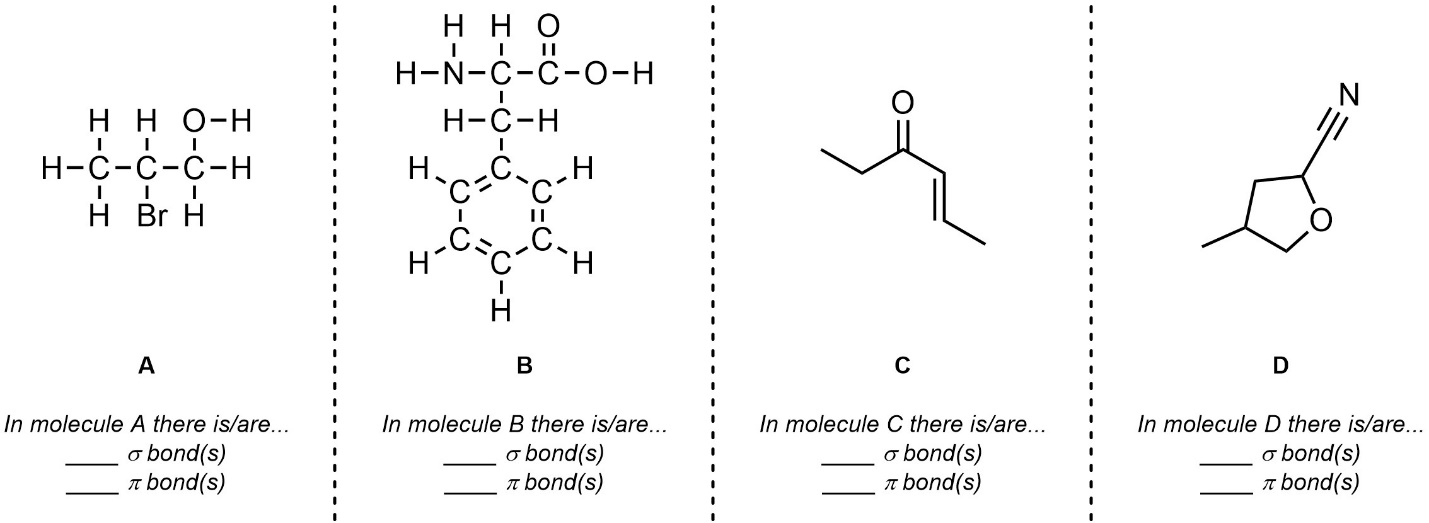
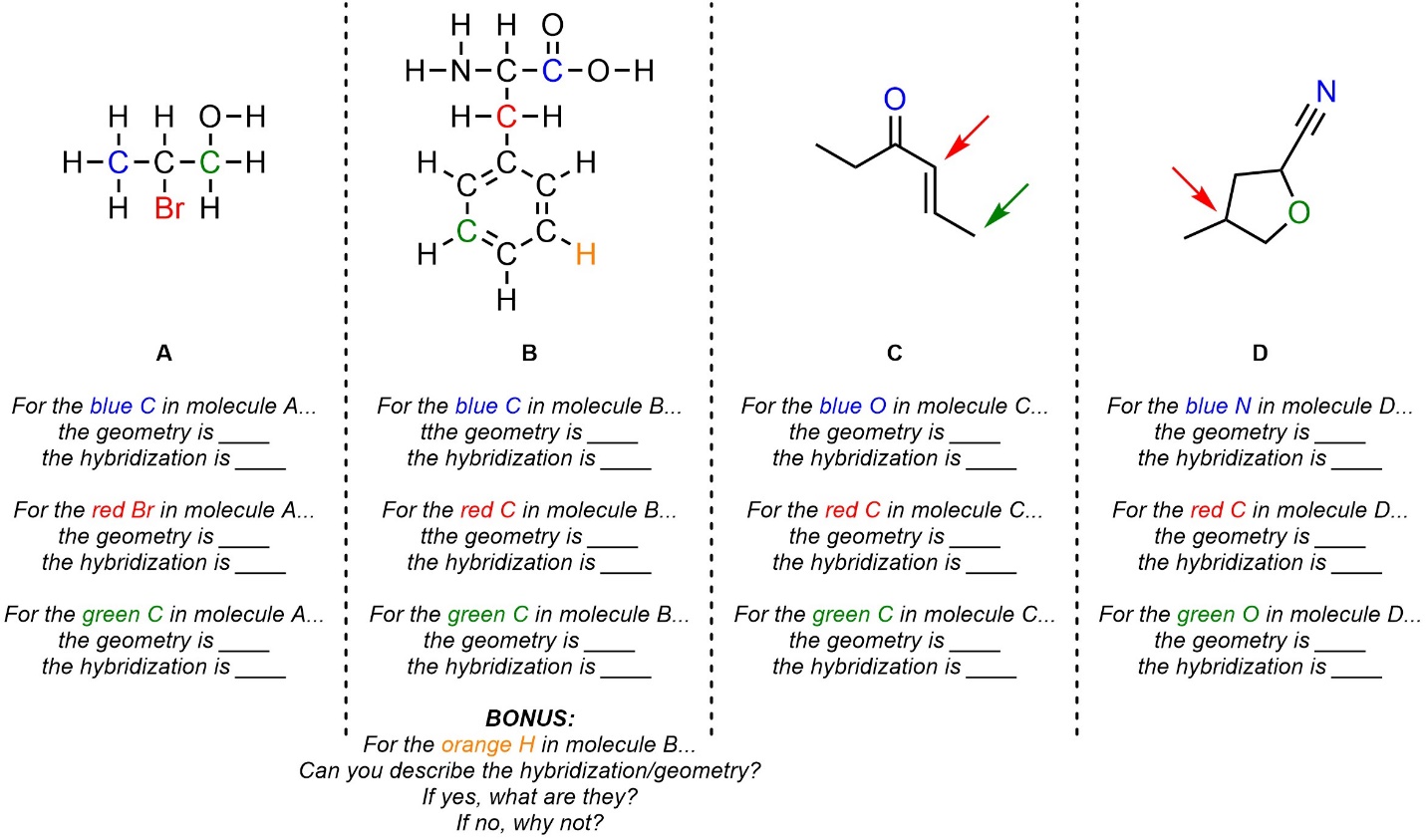
Q1.2: What is the hybridization and geometry around the highlighted atoms in these molecules? If an arrow points to a part of the molecule assume it is pointing to the carbon atom there.
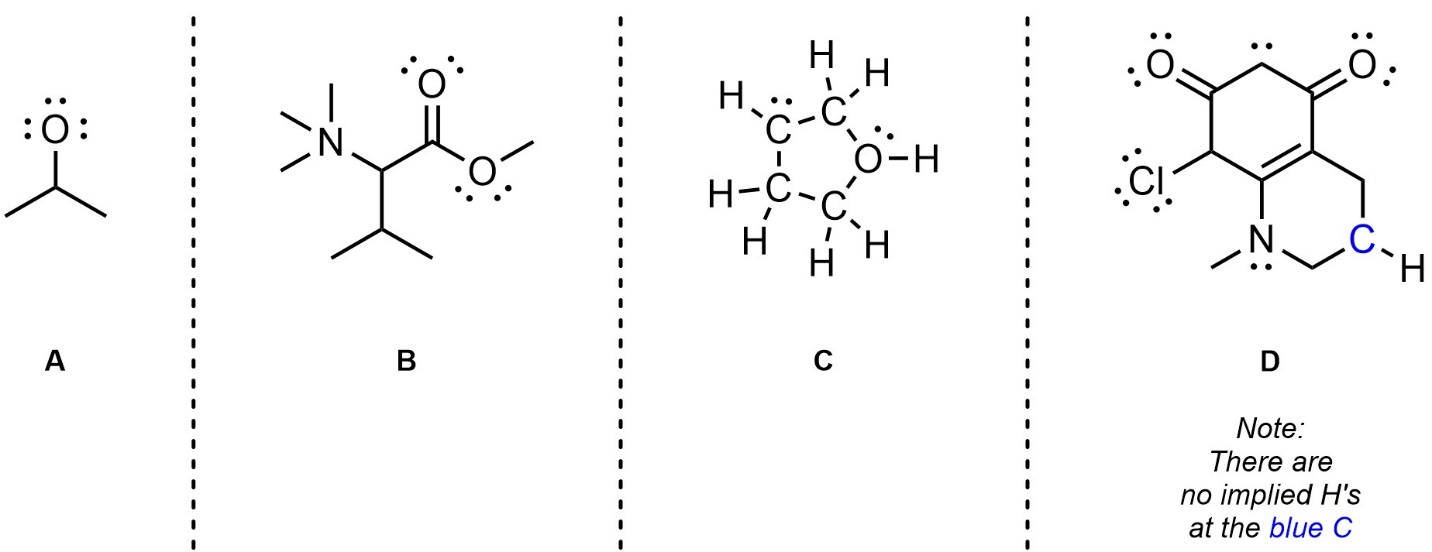
Q1.3: Each molecule below contains at least one atom with a formal charge that is not being shown. For convenience all lone pairs are drawn for you. Redraw each molecule and calculate and add the missing formal charges to the appropriate places.
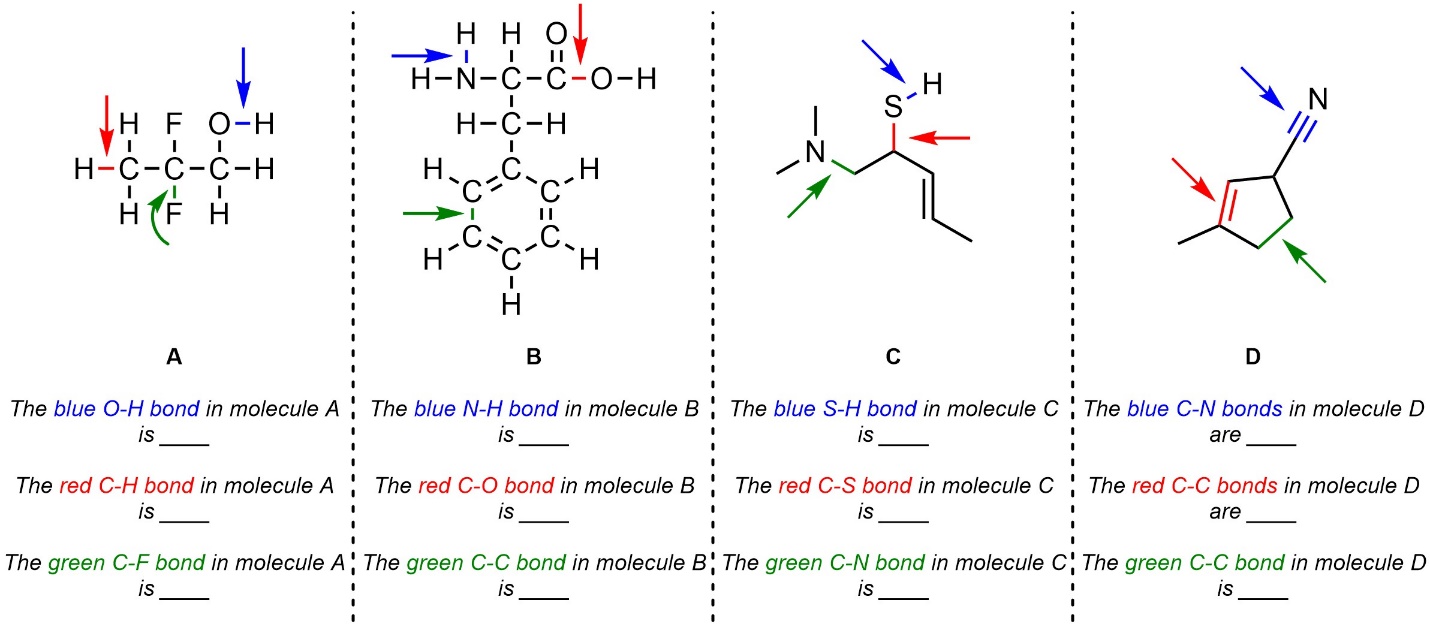
Q1.4: Class each indicated bond as polar or non-polar.
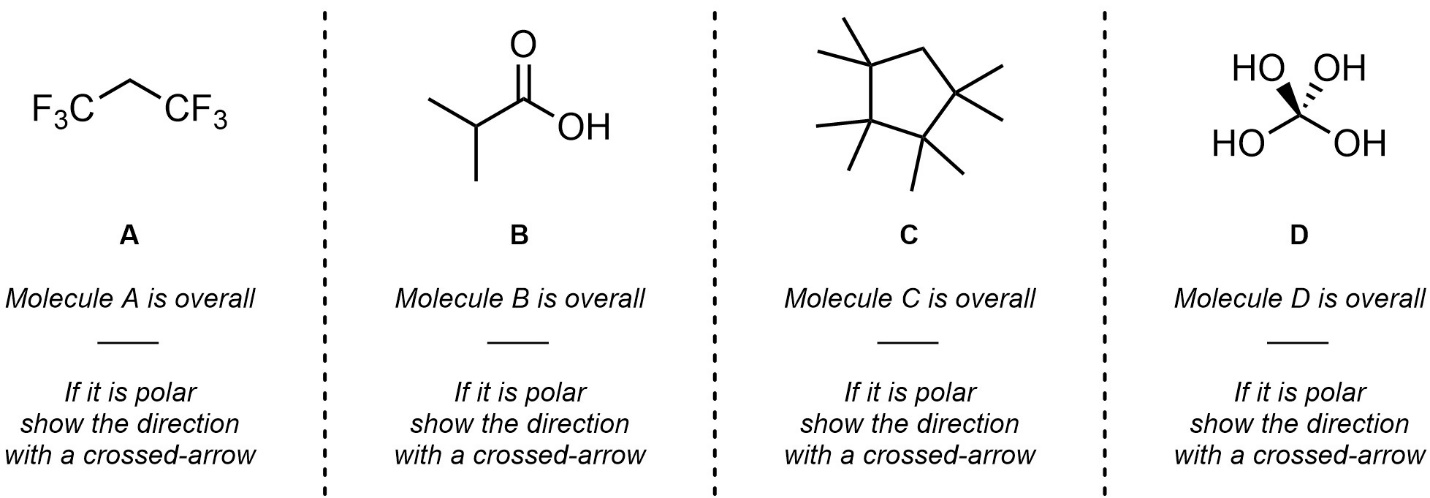
Q1.5: Class each molecule as overall polar or non-polar. If it is polar, indicate the overall direction of polarity (net dipole/dipole moment) with a “crossed arrow”.
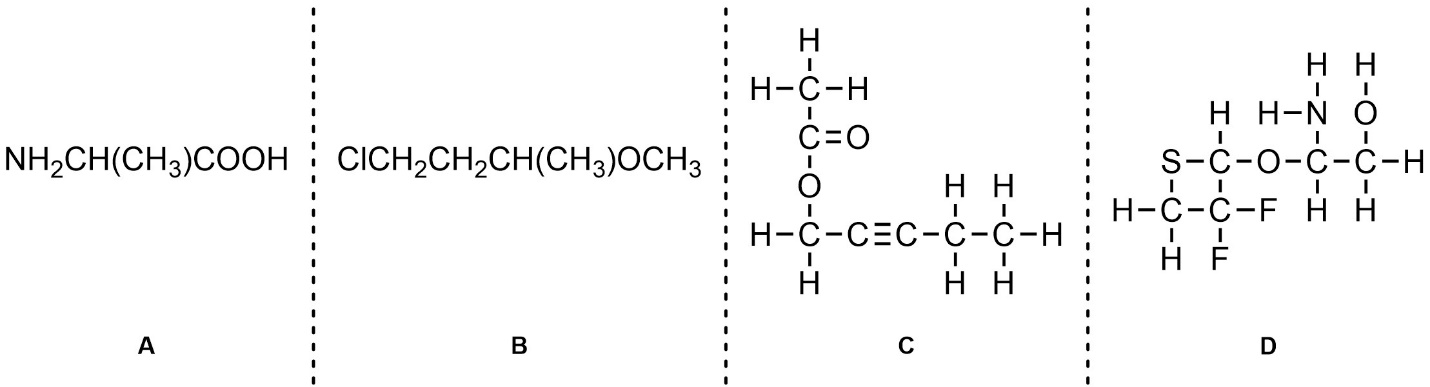
Q1.6: (Re)Draw the following as line-angle structures. Show all lone pairs.
Q1.7: (Re)Draw the following as Lewis structures.