7.8. Stereochemistry of Addition Reactions
Simple additions to symmetrical carbonyl-containing compounds like acetone produce no stereocentres. However, additions to non-symmetrical carbonyls can produce a new stereocentre. For example, a nucleophilic attack onto butan-2-one will produce multiple stereoisomers (Figure 7.17). Specifically, attacking from the top (red arrow; towards the viewer) and attacking from the bottom (blue arrow; away from the viewer) of the ketone will produce a different absolute configuration at a new stereocentre.
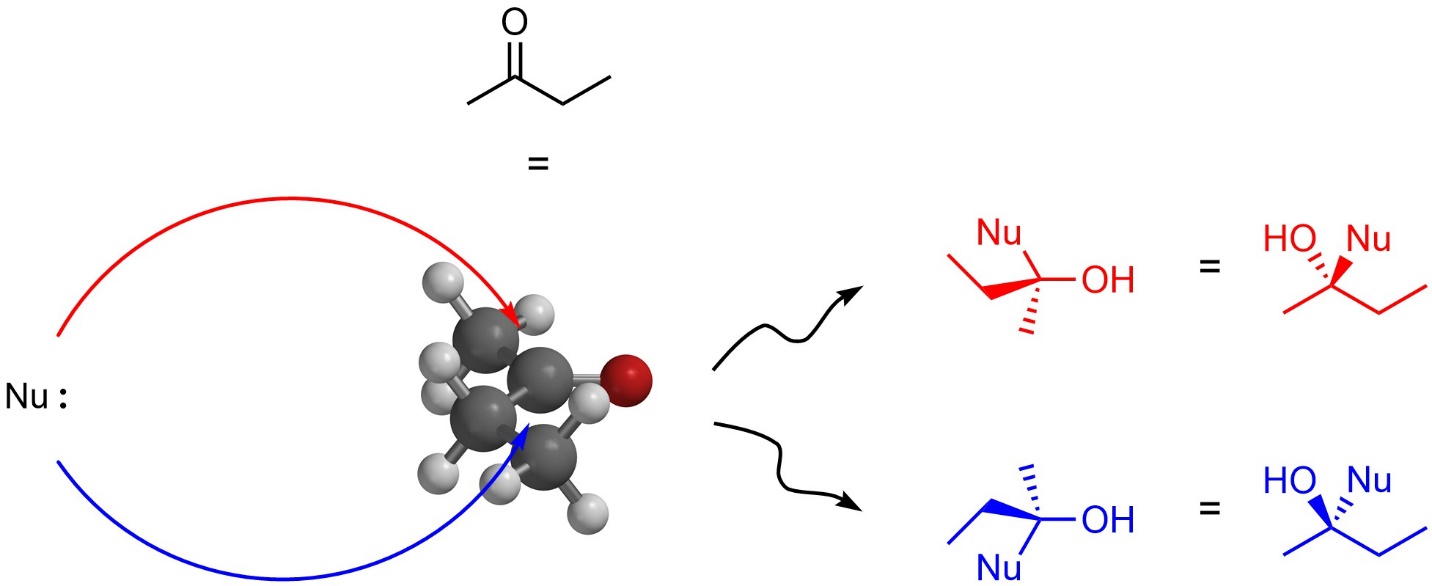
Figure 7.17 – Comparison of Products from Nucleophilic Attacks on the Top and Bottom Sides of Butan-2-one.
In this case only one stereocentre exists (the one that was made) and the result is a pair of enantiomers. Because there is no steric or electronic difference between attacking from the top or bottom in this reaction, the two stereoisomers are formed equally and the product is a racemate. Specific terminology to differentiate these two attacks exists (Re/Si facial selectivity) but is generally considered too advanced for introductory material.
If there is already a stereocentre in the molecule that is being attacked then the relationship between the two stereoisomers formed is diastereomers instead of enantiomers (Figure 7.18). Because there is a steric and/or electronic difference between attacking from the top or bottom in this reaction, the two stereoisomers are NOT formed equally and the product is a non-one-to-one mixture of diastereomers.
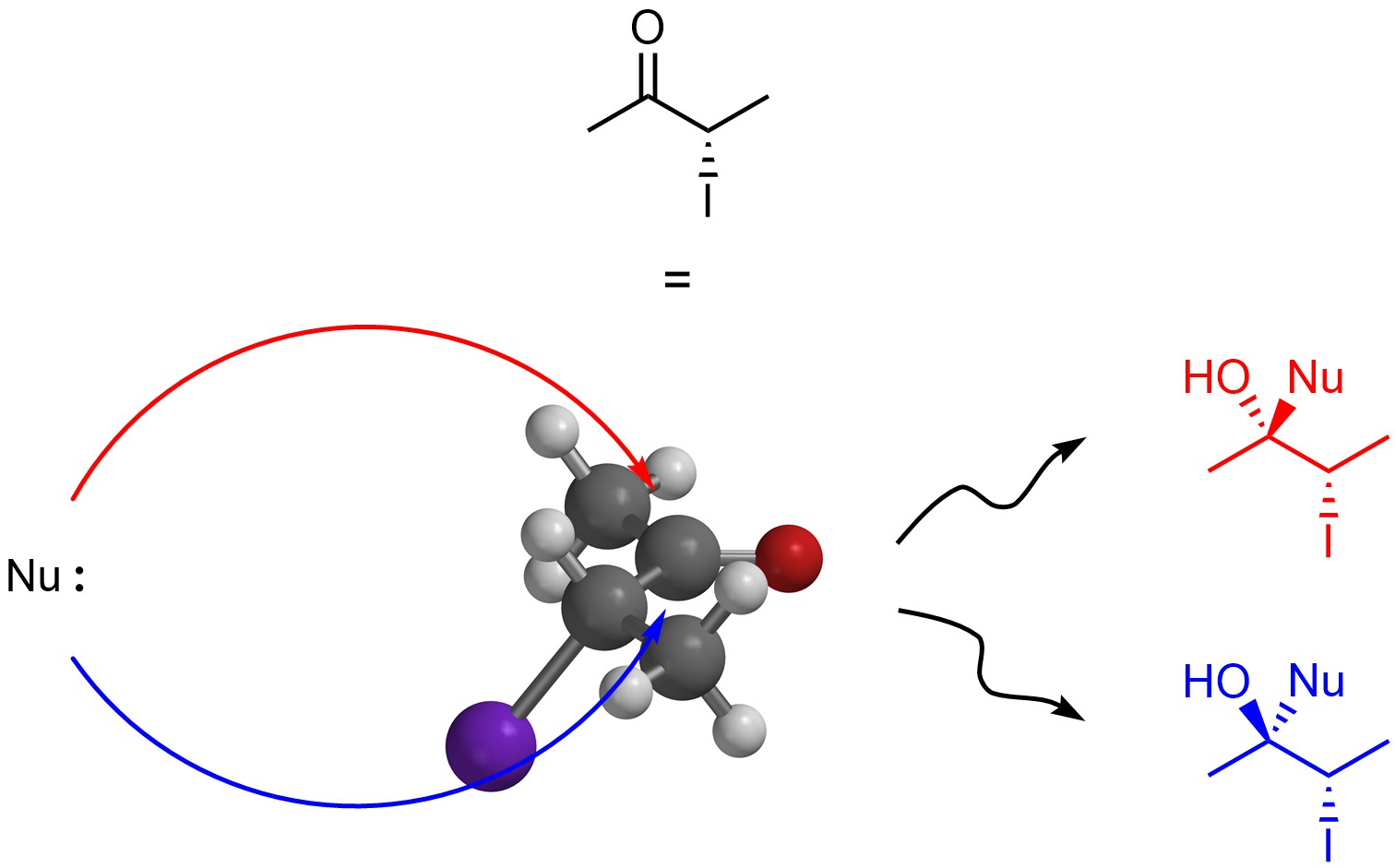
Figure 7.18 – Comparison of Products from Nucleophilic Attacks on the Top and Bottom Sides of (S)-3-iodobutan-2-one.
At an introductory level it is important only to recognize whether the product will have no stereocentres (stereochemistry does not matter), will be formed as a racemate, or will be formed as a mixture of diastereomers. Predicting which diastereomer should be favoured is not required.

