10.10. Regioselectivity and Substituent Effects
Consider an aromatic ring with a pre-existing substituent undergoing an SEAr reaction (Scheme 8.2). There are three different compounds possible as products.
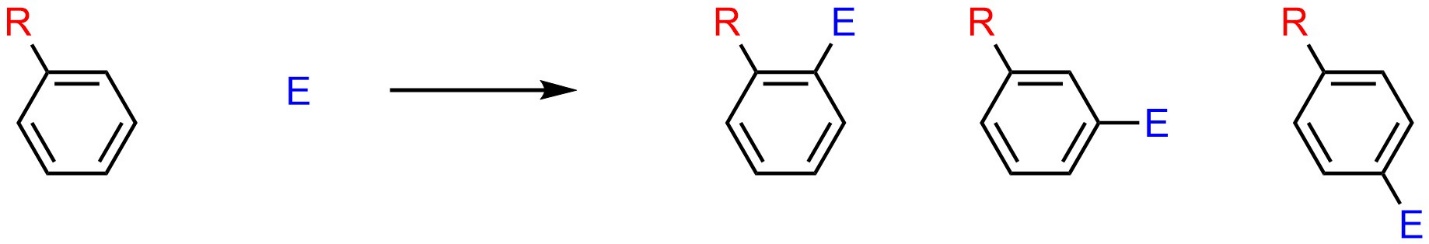
Scheme 10.17 – Possible Regioisomers from Electrophilic Aromatic Substitution of an Aromatic Ring with a Pre-Existing Substituent.
The relationship between these products is constitutional isomers. Specifically, they are regioisomers where the new group has been added ortho, meta, or para to the original group.
It is possible to predict the major regioisomer(s) and whether the reaction will be fast or slow based on the structure of the original group.
10.10.1. Electron-Donating Groups (EDGs) are Activating and o/p-Directing
Any group that adds electron density to the aromatic ring is referred to as an electron-donating group (EDG). Electron density can be added through resonance or hyperconjugation (Figure 10.4). Resonance is by far the more important factor.
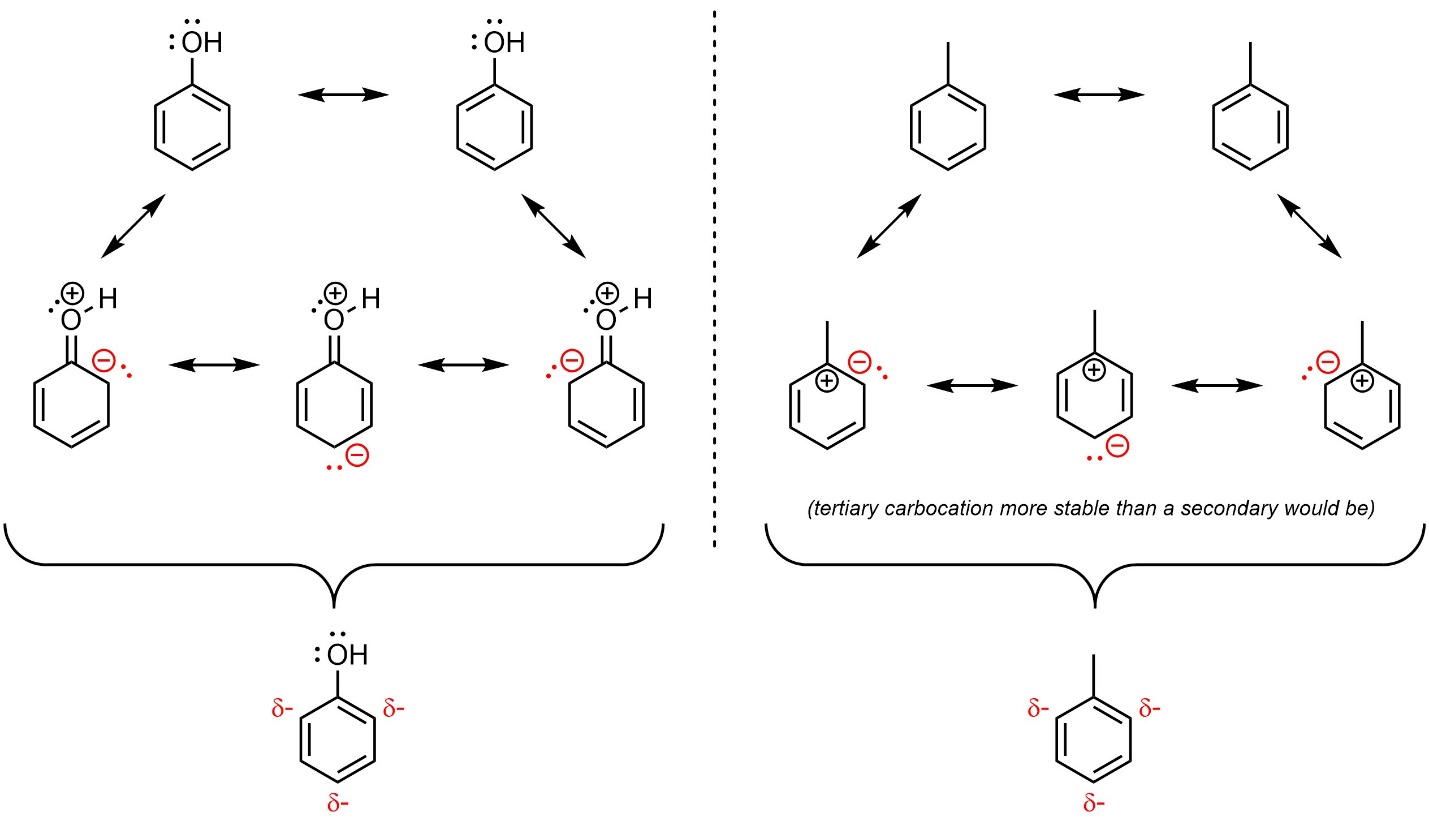
Figure 10.4 – Examples of Aromatic Rings with an Electron-Donating Group.
The rate-determining step for SEAr reactions is the nucleophilic attack of the aromatic ring on the electrophile, generating the carbocation (see Figure 10.1). Adding electron density to the aromatic ring makes it more nucleophilic, which makes this step faster. As a result, electron-donating groups speed up SEAr reactions. This is commonly referred to as being activating or activating the ring.
When electron density is added to the ring through substituents it is localized to the ortho and para positions. This is easiest to see by considering the resonance structures, which show partial anionic charges at these positions (see Figure 10.4). These positions are therefore the most nucleophilic. As a result, electron-donating groups are regioselective for adding new groups ortho and para to themselves. This is commonly referred to as being ortho/para-directing.
The two regioisomers will not be formed in equal amounts, but the relative amount of each varies significantly depending on the exact structure of the electron-donating group. At an introductory level drawing both regioisomers as the major products of the reaction is acceptable.
10.10.2. Electron-Withdrawing Groups (EWGs) are Deactivating and m-Directing
Any group that removes electron density from the aromatic ring is referred to as an electron-withdrawing group (EWG). Electron density can be removed through resonance or induction (Figure 10.5). Resonance is by far the more important factor.
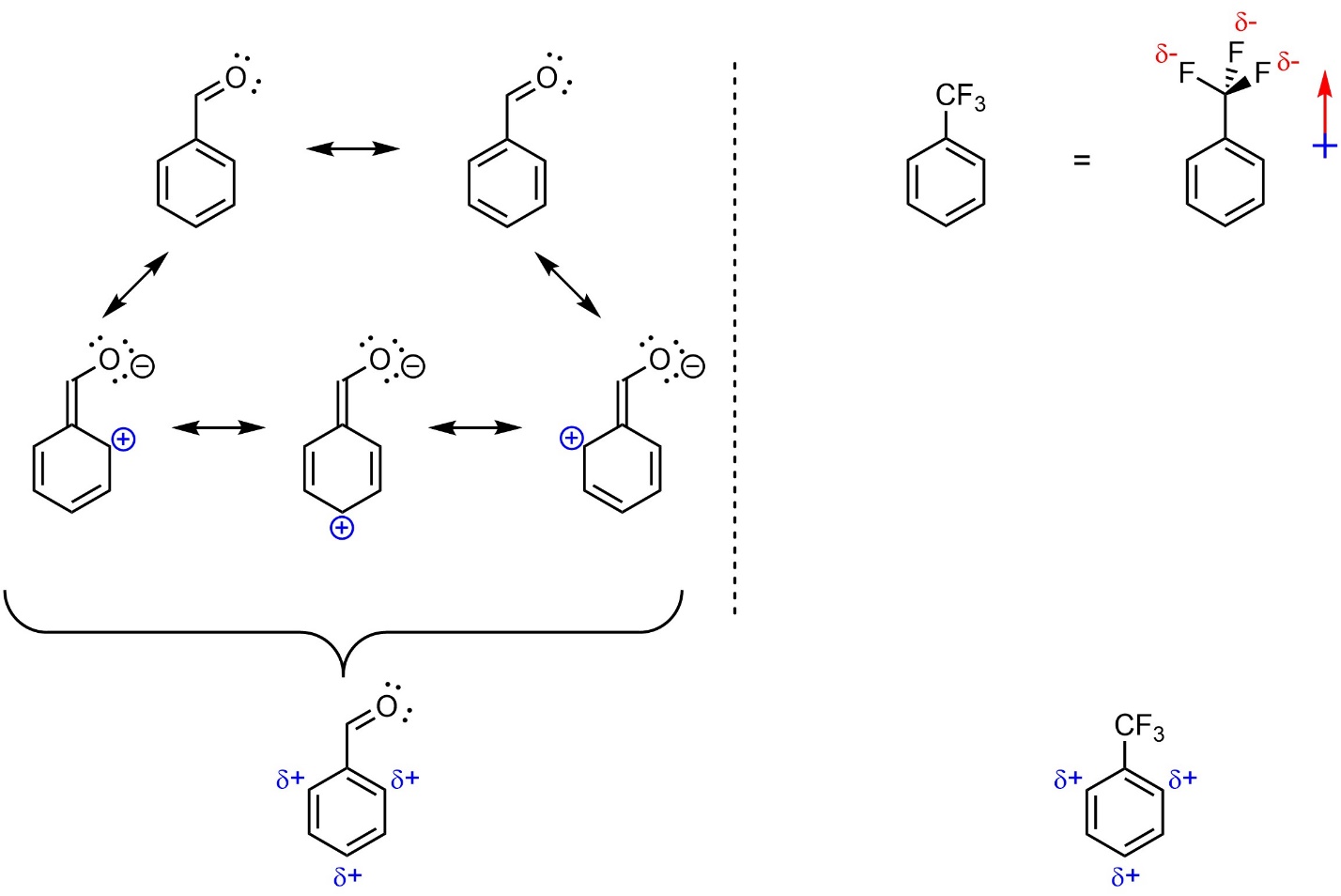
Figure 10.5 – Examples of Aromatic Rings with an Electron-Withdrawing Group.
The rate-determining step for SEAr reactions is the nucleophilic attack of the aromatic ring on the electrophile, generating the carbocation (see Figure 10.1). Removing electron density from the aromatic ring makes it less nucleophilic, which makes this step slower. As a result, electron-withdrawing groups slow down SEAr reactions. This is commonly referred to as being deactivating or deactivating the ring.
When electron density is removed from the ring through substituents it is removed primarily from the ortho and para positions. This is easiest to see by considering the resonance structures, which show partial cationic charges at these positions (see Figure 10.5). This is also true for groups which remove electron density through induction. Unfortunately, a discussion of why the electron density from these positions is selectively reduced by induction is beyond the scope of this text. These positions are therefore the least nucleophilic. As a result, electron-withdrawing groups are regioselective for adding new groups meta to themselves. This is commonly referred to as being meta–directing.
10.10.3. Exception: Halides
One group of substituents breaks this pattern. Halides (X = F, Cl, Br, I) are electron-withdrawing through induction, which makes them deactivating (Figure 10.6). However, instead of being meta-directing they are ortho/para-directing. The change in regioselectivity is due to the presence of lone pairs, which donate to the ring very slightly.
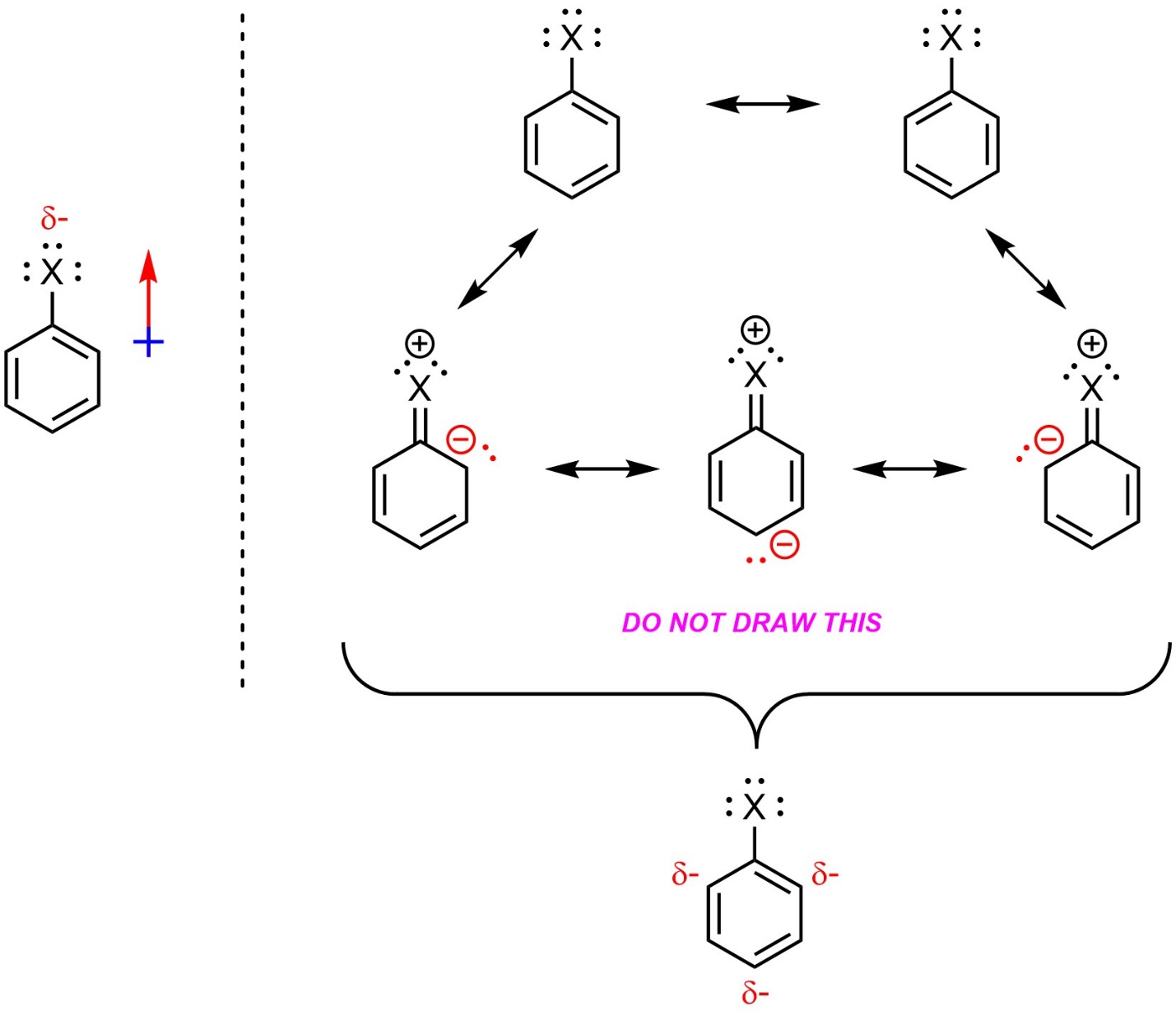
Figure 10.6 – Generalized Example of an Aromatic Ring with a Halide Group.
This image is provided only to help rationalize the regioselectivity for halide groups. Do not draw resonance structures using the lone pairs of halides in any other context.
10.10.4. How to Determine if a Group is an EDG or an EWG
Any group that can participate in resonance with the aromatic ring is trivially easy to class. If resonance is not possible, then induction is checked. If neither of these factors is present hyperconjugation is considered. The halides are the only exception requiring memorization.
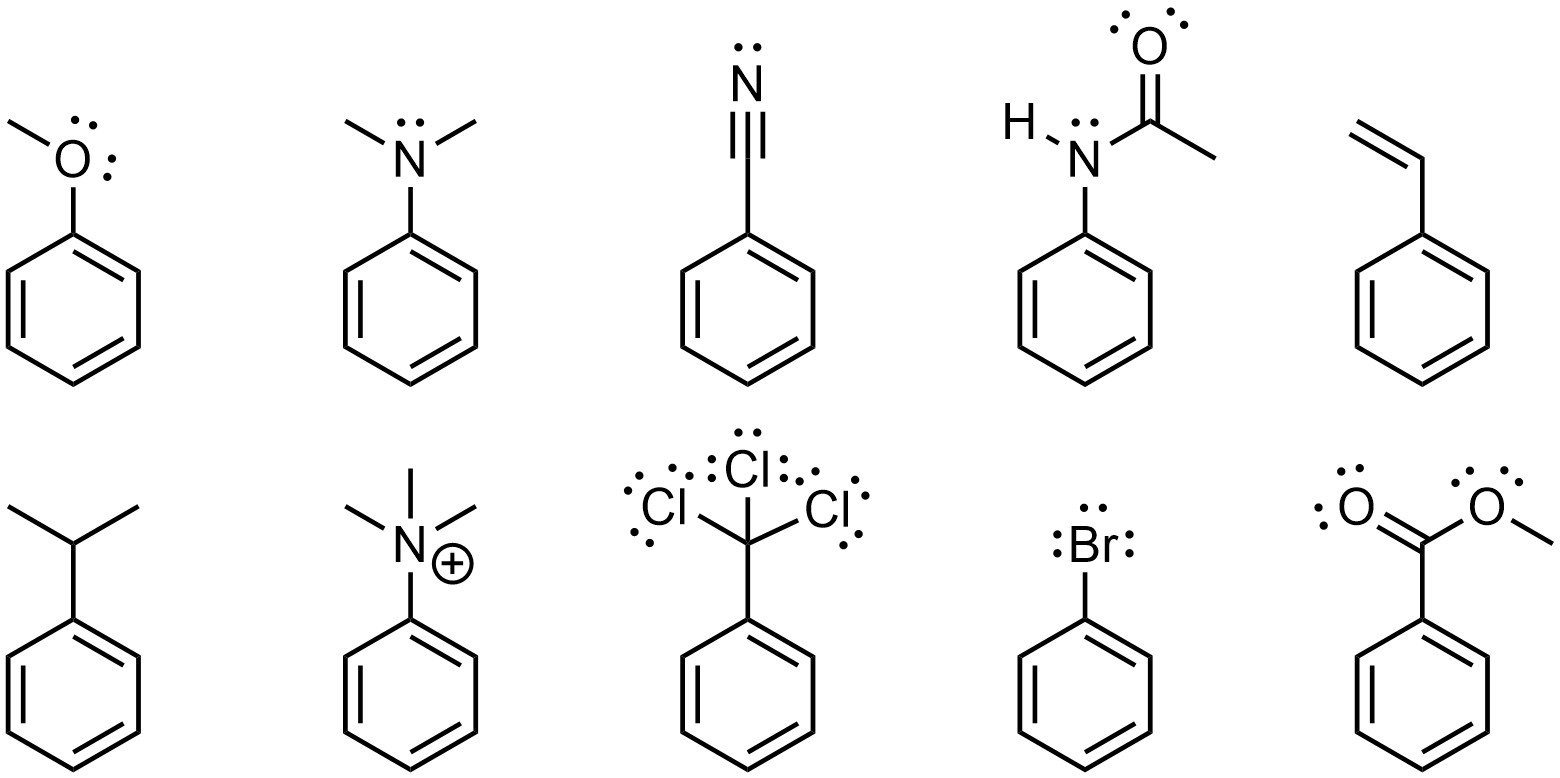
- Resonance. If a resonance structure can be constructed pushing electron density into the ring, it is an electron-donating group. If a resonance structure can be constructed pulling electron density out of the ring, it is an electron-withdrawing group. If both are possible, electron-donation is (usually) preferred. It is not required to draw the full resonance structure(s), only to recognize if they are possible.
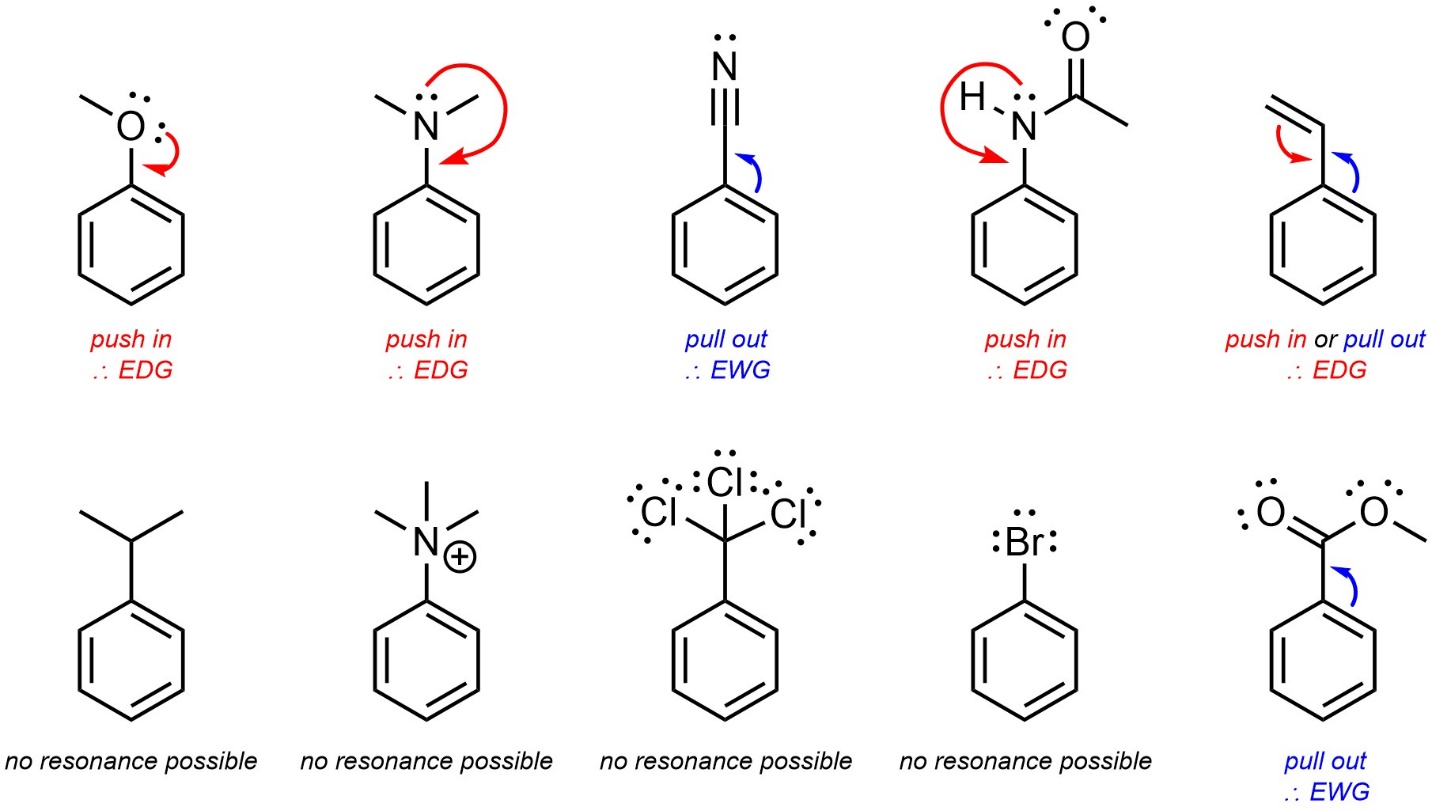
- Induction. Induction through one or more electronegative atoms is then checked. Inductive groups are electron-withdrawing unless already classed from resonance. A cationic charge affects the strength of induction (see Section 10.10.5.) but has no other effect. Remember that halides are electron-withdrawing but have altered regioselectivity.
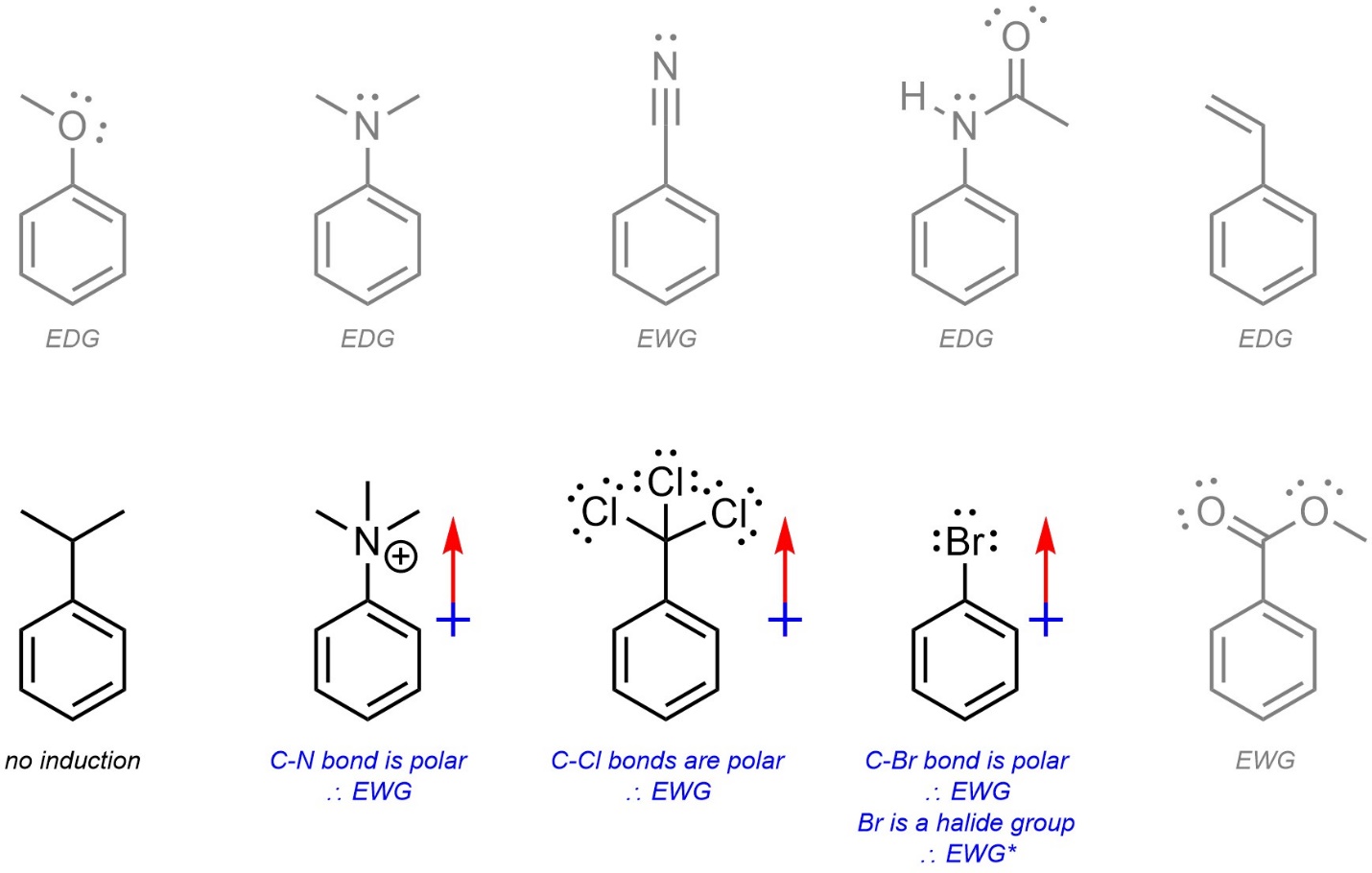
- Hyperconjugaton. Alkyl groups are electron-donating groups through hyperconjugation (stabilization of adjacent carbocation).
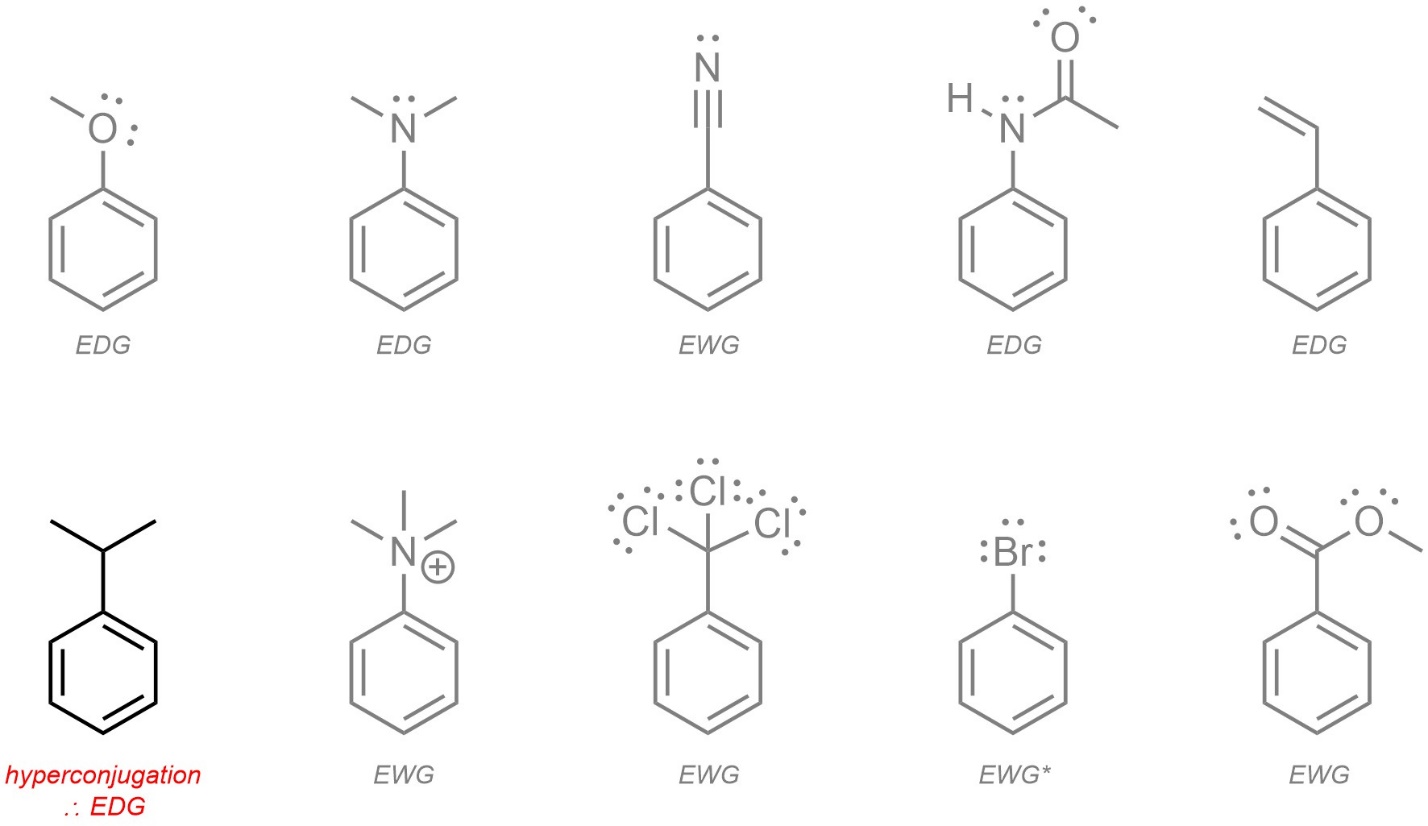
The initial step, checking for resonance, is occasionally problematic for students. The only consideration is whether or not a resonance form can be constructed that pushes in or pulls out electron density to/from the aromatic ring. Additional resonance outside of the ring affects the strength of donation (see Section 10.10.5.) but does not affect whether it is electron-donating or electron-withdrawing.
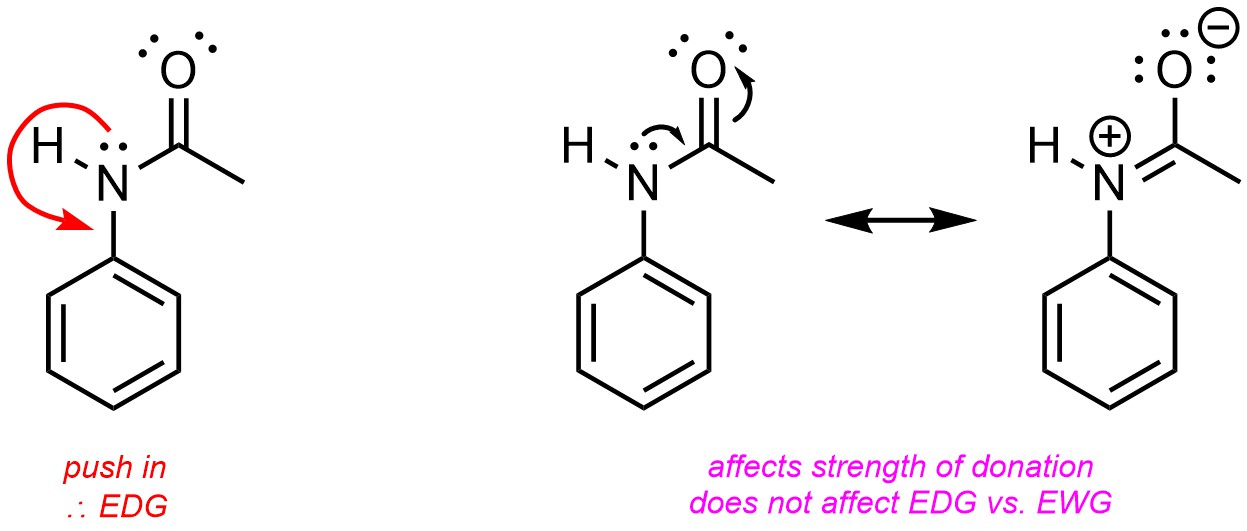
10.10.5. How to Qualitatively Compare EDGs and EWGs
It is possible to quantify electron-donating and electron-withdrawing ability. However, this relies on empirical values that are not measurable for all substituents. Instead, qualitative comparisons are commonly made. Many comparisons are too difficult to definitively determine at an introductory level; this text will focus on differences which are straightforward to compare.
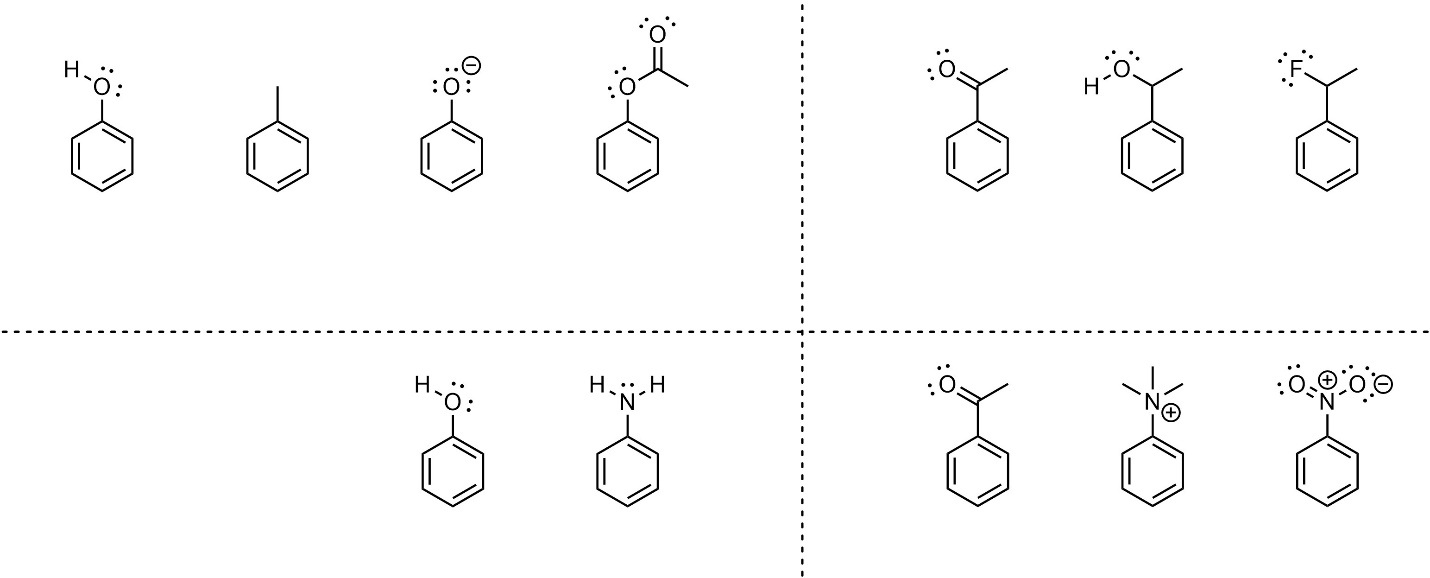
- Charges (anions/cations) directly adjacent to the ring. Anionic electron-donating groups are stronger donators than all other groups. Cationic electron-withdrawing groups are stronger withdrawers than all other groups. This effect is stronger than resonance.
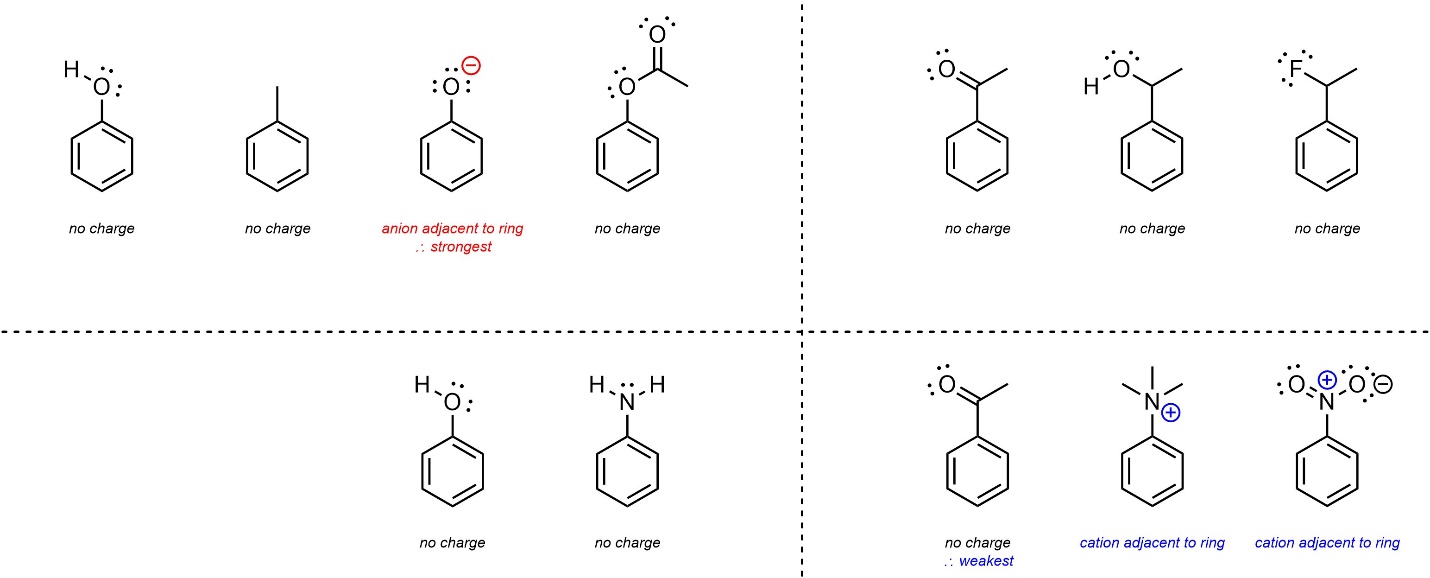
- Resonance. Groups that donate through resonance are stronger than those that do not. Groups that withdraw through resonance are stronger than those that do not. Groups that donate/withdraw from the ring through resonance but also have resonance delocalization outside of the ring donate/withdraw less than those that do not.
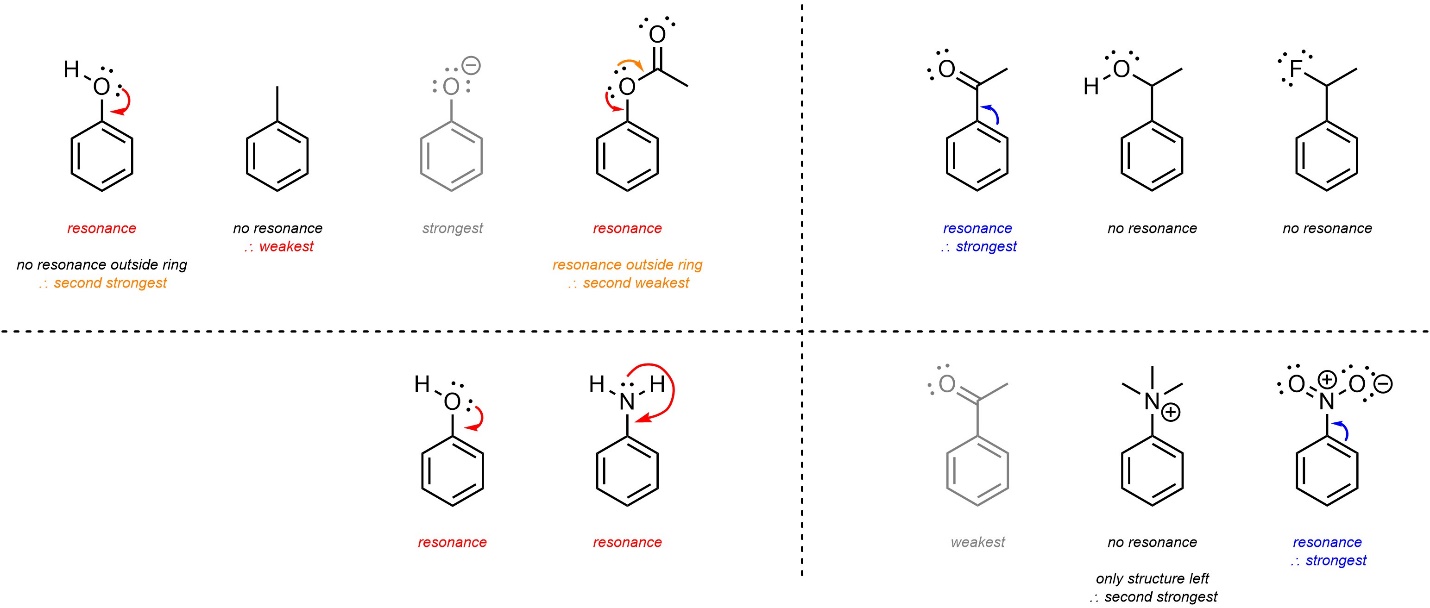
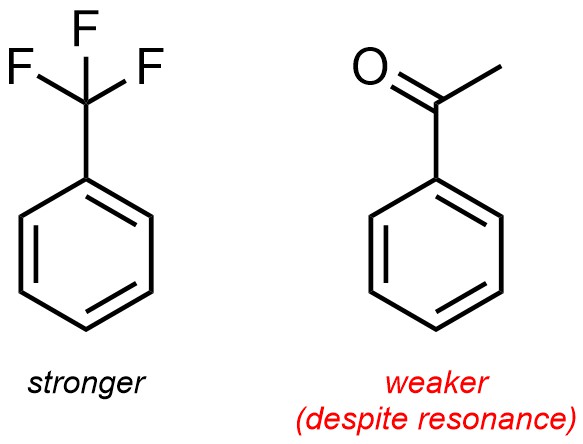
- Induction/Electronegativity. All remaining groups are compared based on their relative induction (electronegativity, proximity, and number of atoms). Stronger induction weakens donation and strengthens withdrawal.
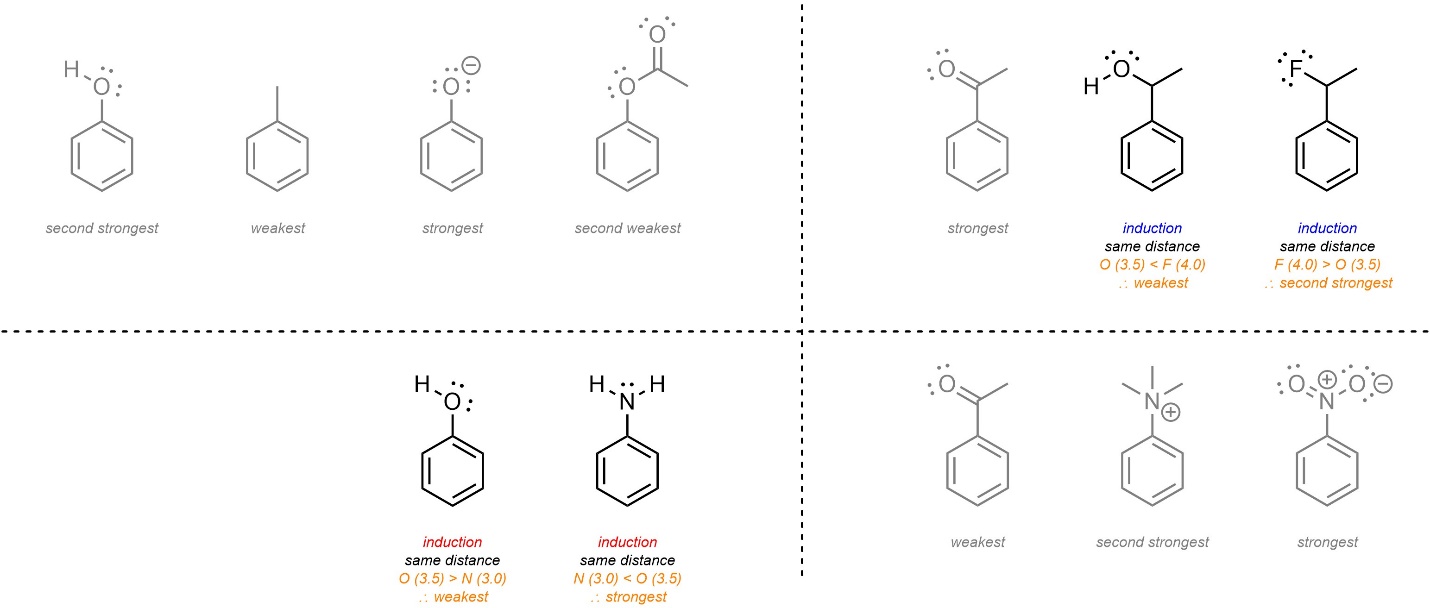
This approach will effectively compare most sets of electron-withdrawing or electron-donating groups. There are exceptions to this pattern. For example, the CF3 group is actually a stronger electron-withdrawing group than an adjacent carbonyl (such as a ketone), despite resonance normally being a more important factor than induction. At an introductory level memorizing exceptions is not required.
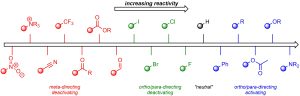
The following is provided for quick referencing. It is not necessary to memorize this chart, it is more important to be able to qualitatively compare groups.
10.10.6. How to Determine Regioselectivity With Multiple Pre-Existing Groups
It is possible for there to be multiple pre-existing groups on an aromatic ring. When this occurs it may, or may not, require extra consideration. The general approach is to consider which regioisomer(s) will be formed the fastest.
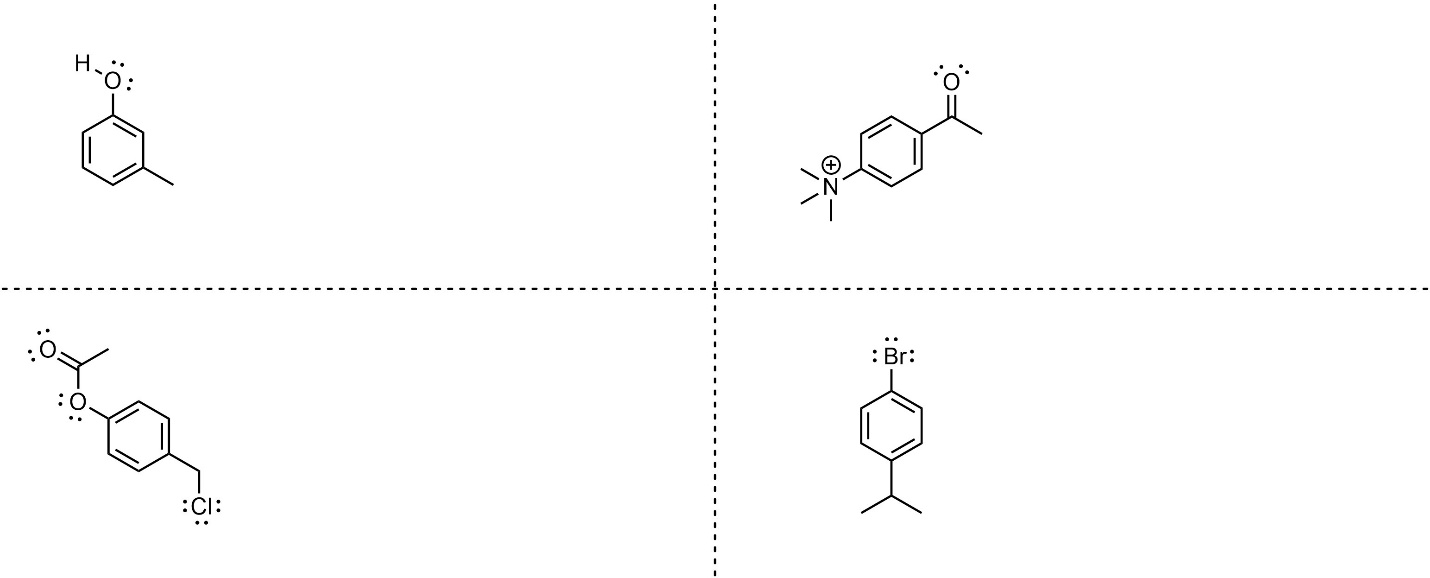
- Determine where each group is regioselective for. Follow the standard process for each group. Remember that if a group already exists at a selected-for position it will not be possible to add a new group there without breaking aromaticity.
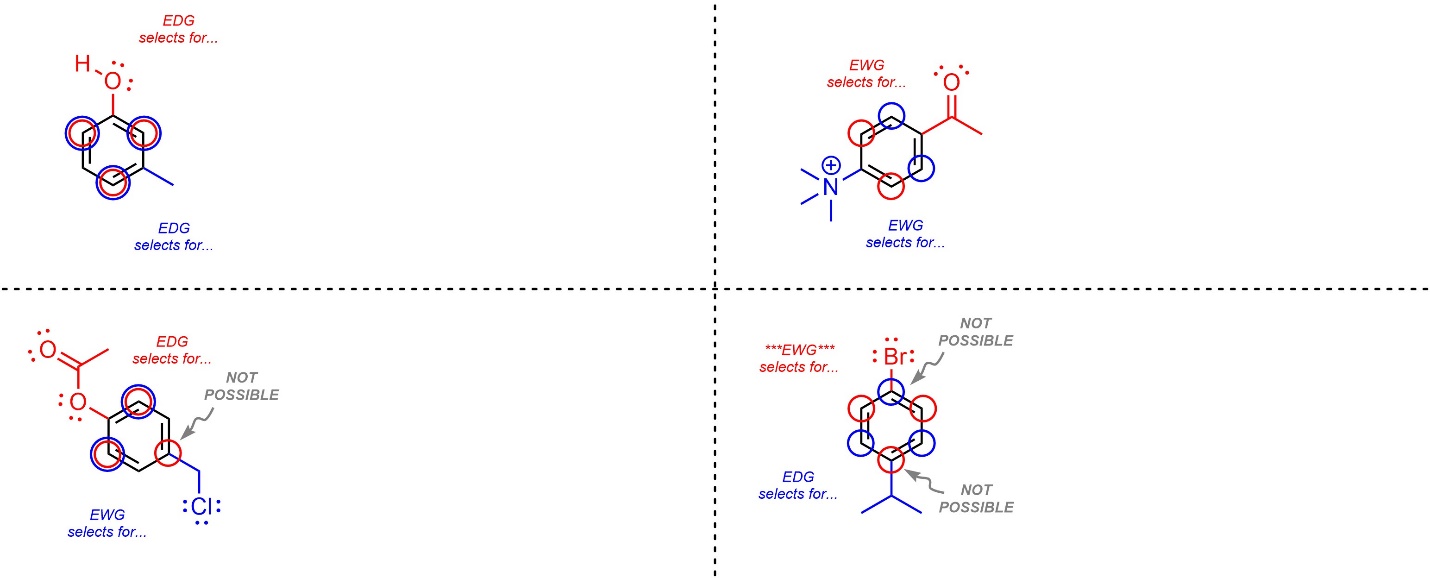
- Check for matching regioselectivity. If all of the groups select for the same regioisomer(s), then no further consideration is needed. As always, only draw multiple products if they are different compounds.
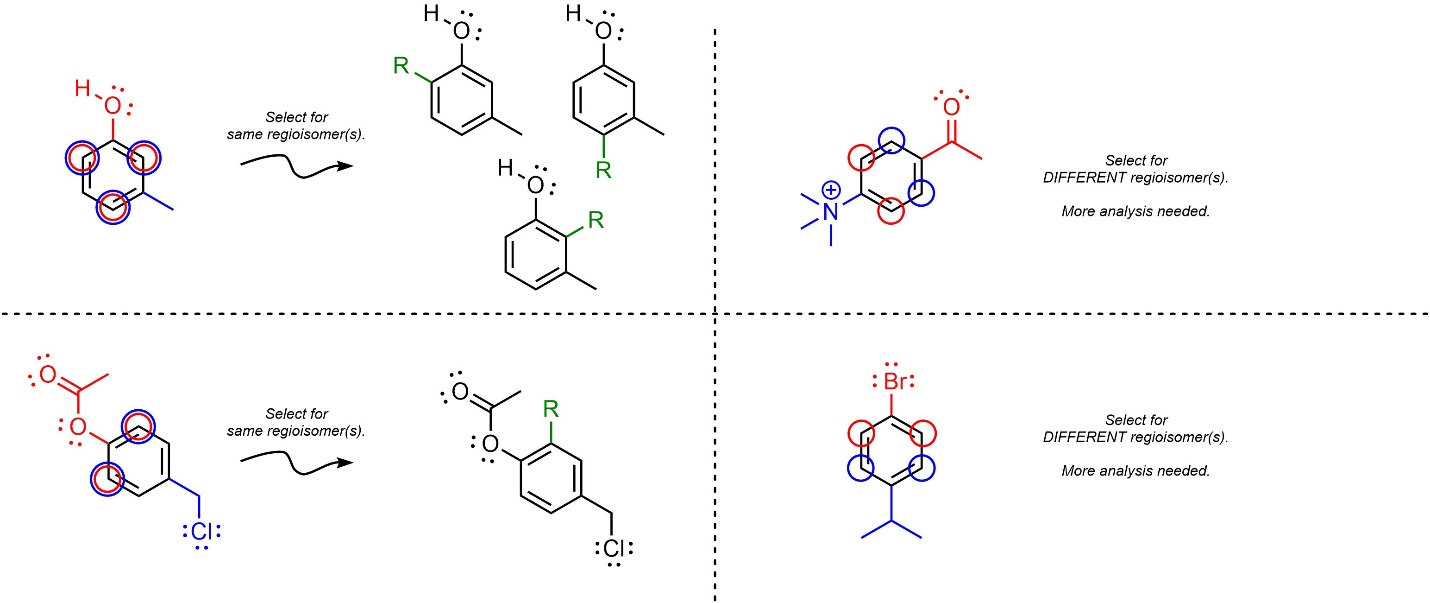
- Determine which product(s) would be formed fastest. Because SNAr reactions are (functionally) irreversible, whatever regioisomer(s) form fastest will be the major product(s). Determine which group is the most activating (or LEAST deactivating) group and use it to determine the regioselectivity. Compare groups using the standard procedure.
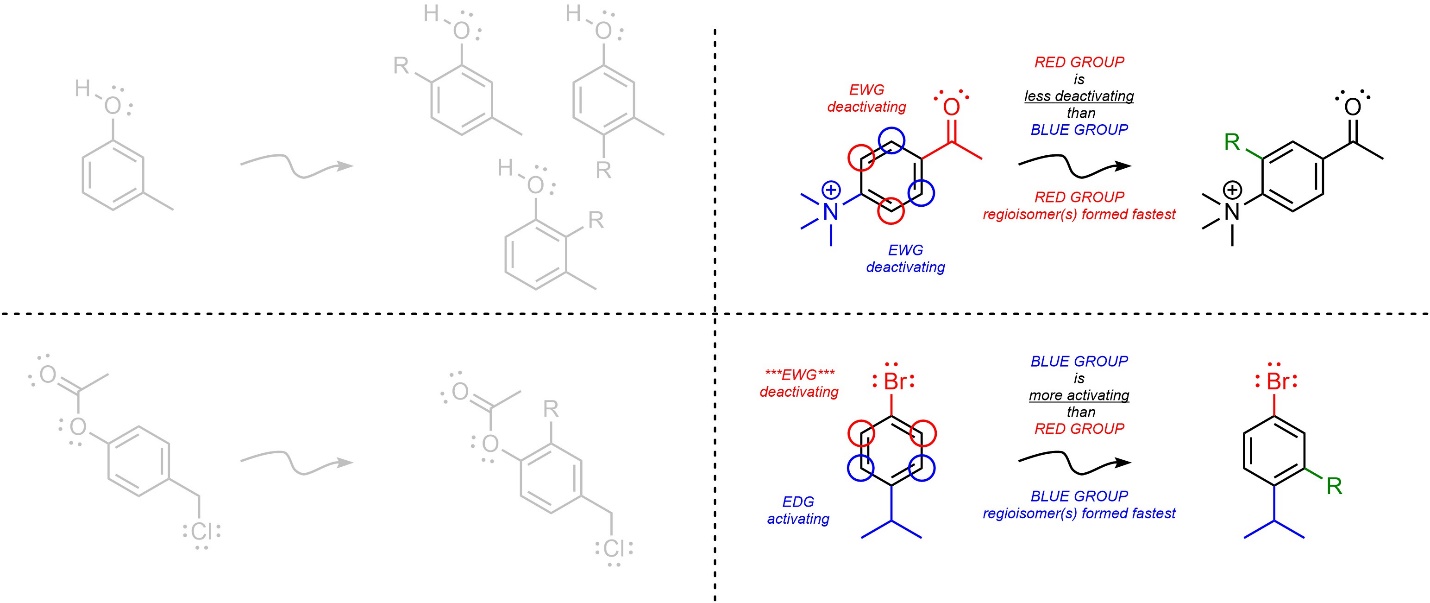
Variations on this idea, such as molecules with multiple aromatic rings with different groups undergoing an SNAr reaction, can also be considered. The same general approach, determining which regioisomer(s) are formed fastest, will also work in these cases.

