Chapter 6 Practice Problems – Answers
Q6.1: Determine the most and least acidic H’s in each set. The H’s you should compare are highlighted. For practice purposes you should do this twice: first without looking at any pKa values (qualitatively), then again using values from the pKa tables provided in the textbook (quantitatively).
Being fast with qualitative comparisons requires practice.
Creativity with the tables of pKa values may be required.
The two examples highlighted in red are
EXTREMELY difficult (functionally impossible)
to answer qualitatively based on introductory considerations.
They are included to show that
even masters of qualitative comparison must sometimes check pKa tables.
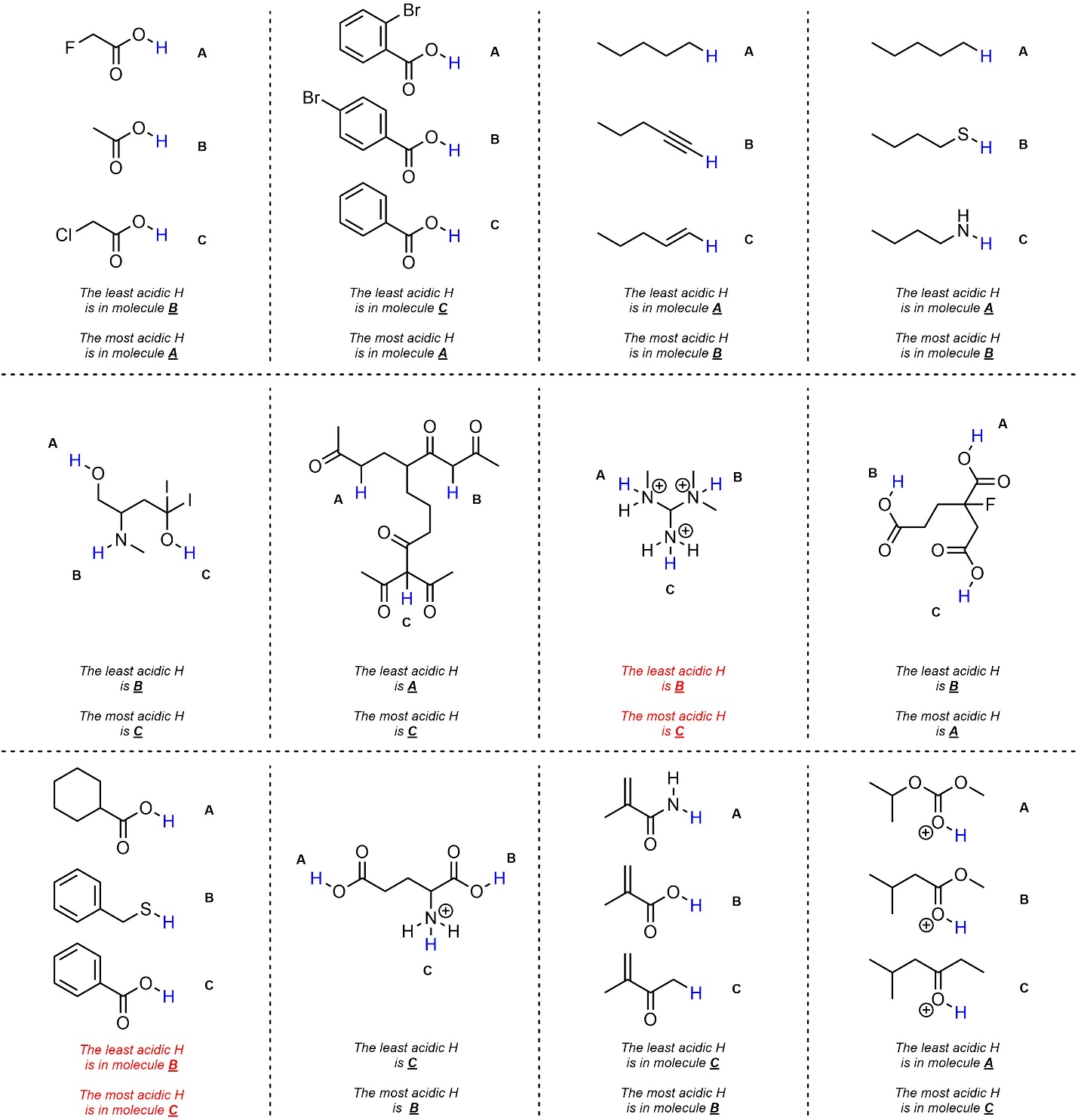
Qualitative answers…
…should consider resonance stabilization first (strongest effect)
…very often rely on considering electronegativity and/or induction.
…(USUALLY) rely on atomic size only if sulfur is involved.
…rely on hybridization considerations infrequently.
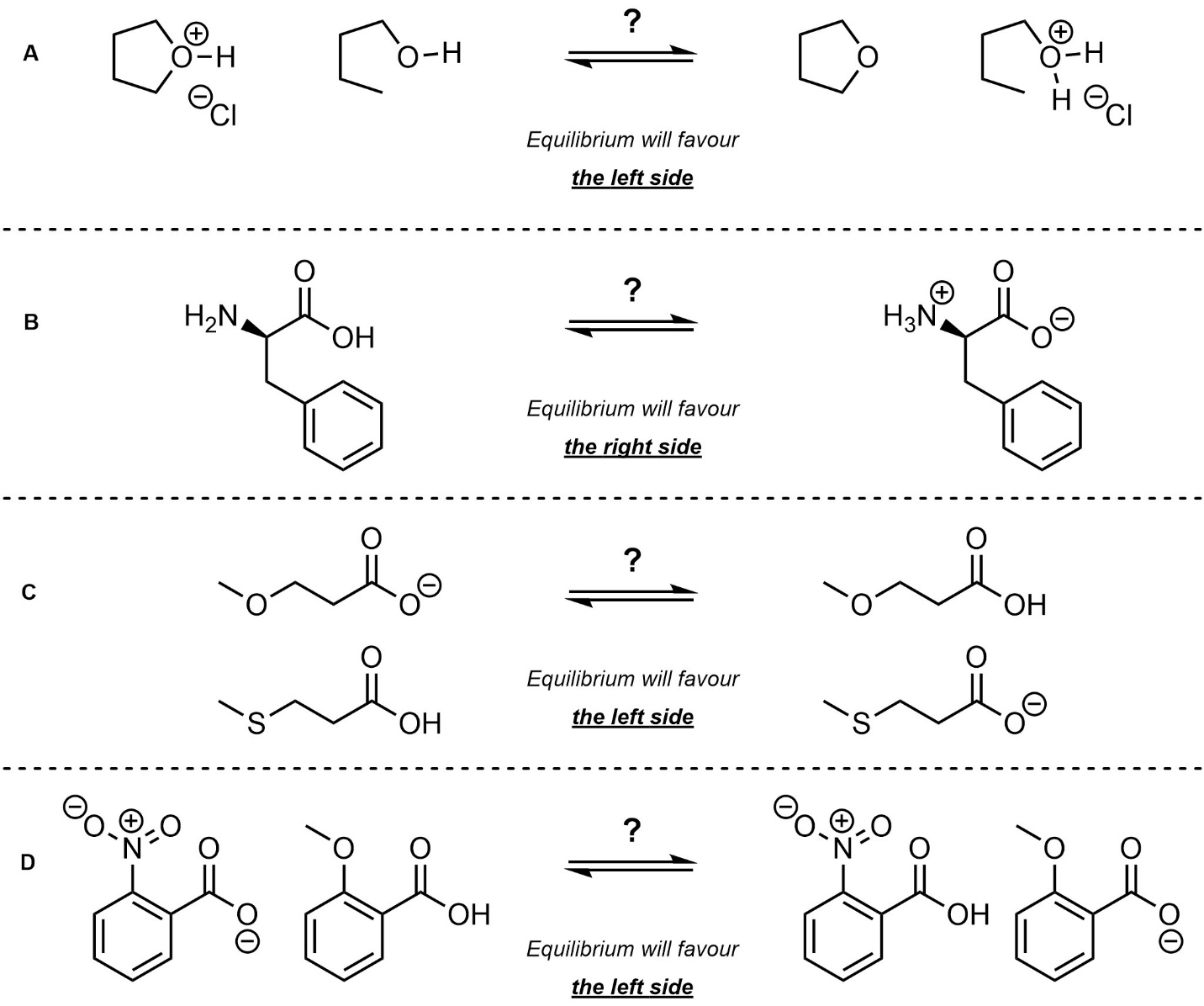
Q6.2: Determine which side of the equilibrium is favoured. For practice purposes you should do this twice: first without looking at any pKa values (qualitatively), then again using values from the pKa tables provided in the textbook (quantitatively).
C is easy to answer qualitatively but hard to answer quantitatively
(to answer qualitatively consider induction/electronegativity)
D is hard to answer qualitatively but easy to answer quantitatively
(to answer qualitatively draw all resonance forms of each acid)
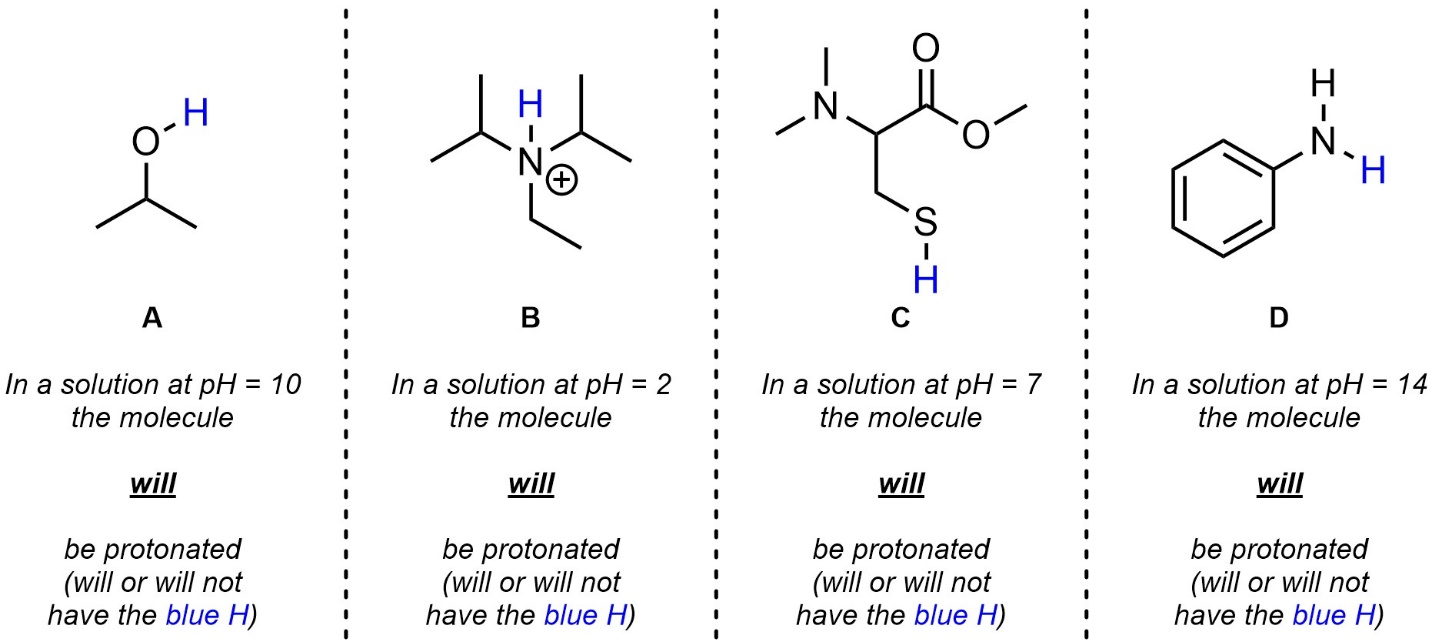
Q6.3: Determine the protonation state of the molecule given the pH of the solution it is in (i.e. whether the molecule primarily exists as shown or as the conjugate base). The H’s you should consider are highlighted. For practice purposes you should attempt this twice: first without looking at any pKa values (qualitatively), then again using values from the pKa tables provided in the textbook (quantitatively).
Note: Because you are comparing to a specific value doing this qualitatively is more challenging; use what you learned/saw from answering the previous questions (qualitatively and quantitatively) to make an informed qualitative choice. Do not be discouraged if your qualitative choice is incorrect as long as you attempted it to the best of your ability.
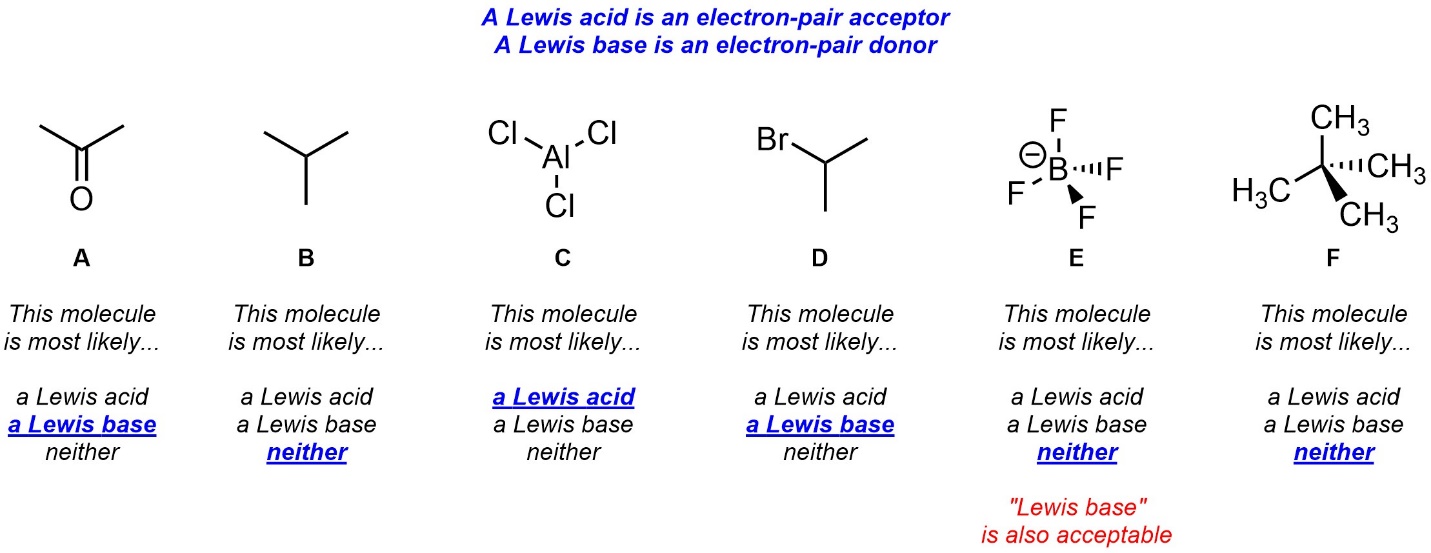
Q6.4: Strictly from memory (without looking up the definitions, searching online, etc.) give a very brief definition of Lewis acid and Lewis base and then class each of the following molecules.