13.5. Reactions with Acid Halide Electrophiles
Acid halides are the most electrophilic of the common functional groups. As a result, they are capable of undergoing many possible reactions.
Theoretically all acid halides are able to undergo all of these reactions. In practice most chemists use acid chlorides much more frequently than the other acid halides. This is due to convenience rather than any major difference in reactivity (see Section 13.7.4).
13.5.1. Reaction: Converting Acid Halides to Carboxylic Acids/Esters
It is possible to convert an acid halide into a carboxylic acid or ester. This can be done using neutral nucleophiles like water (H-OH) or alcohols (H-OR) but it is much more common to use anionic ones such as sodium hydroxide (NaOH) or a sodium alkoxide (NaOR; Scheme 13.4).
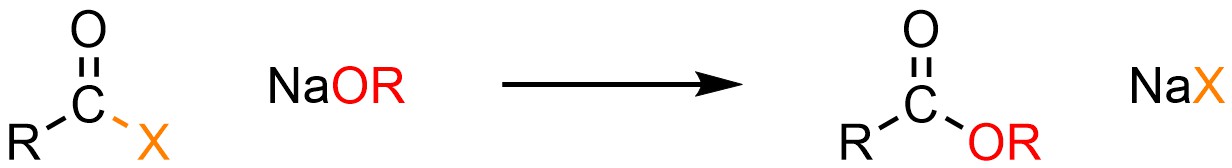
Scheme 13.4 – Generalized Reaction Equation for Addition-Elimination Converting Acid Halides to Carboxylic Acids or Esters.
13.5.1.1. Mechanism
This reaction follows the standard two step mechanism for addition-elimination reactions (see Section 13.3; Scheme 13.5). The nucleophile (alkoxide) attacks the electrophile (acid halide). This forms a new bond and adds a lone pair to the oxygen. Then that lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group (halide).
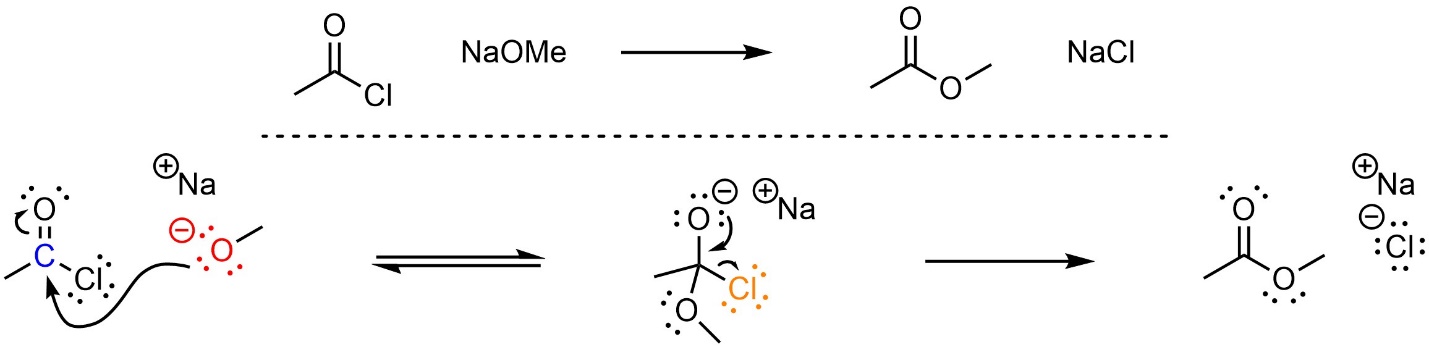
Scheme 13.5 – Reaction Mechanism for Addition-Elimination (Acylation) of Sodium Methoxide with Ethanoyl Chloride.
13.5.2. Reaction: Converting Acid Halides to Anhydrides
It is possible to convert an acid halide into an anhydride. This can be done using neutral nucleophiles like carboxylic acids (H-O(CO)R) but it is much more common to use anionic ones such as sodium carboxylates (NaO(CO)R; Scheme 13.6).
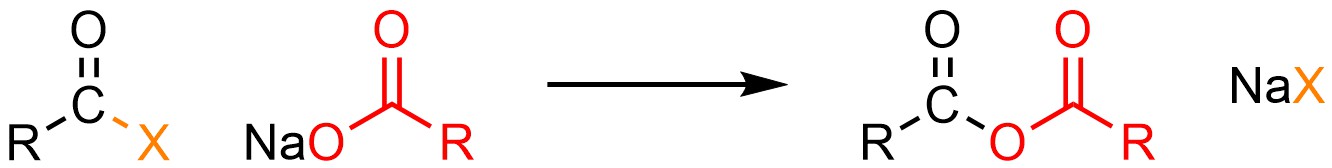
Scheme 13.6 – Generalized Reaction Equation for Addition-Elimination Converting Acid Halides to Anhydrides.
13.5.2.1. Mechanism
This reaction follows the standard two step mechanism for addition-elimination reactions (see Section 13.3; Scheme 13.7). The nucleophile (carboxylate) attacks the electrophile (acid halide). This forms a new bond and adds a lone pair to the oxygen. Then that lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group (halide).
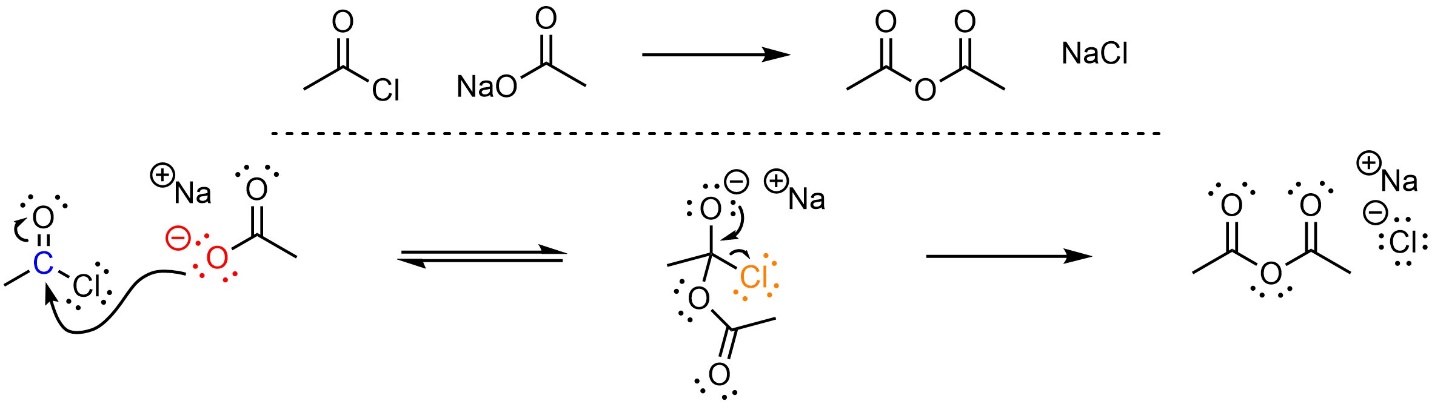
Scheme 13.7 – Reaction Mechanism for Addition-Elimination (Acylation) of Sodium Ethanoate with Ethanoyl Chloride.
13.5.3. Reaction: Converting Acid Halides to Amides
It is possible to convert an acid halide into an amide. Anionic nitrogen atoms have unusual reactivity: they are much more basic than they are nucleophilic. Why this is true is beyond the scope of this text. As a result of their unusual reactivity, this can only be done using neutral nucleophiles like amines (H-NR2; Scheme 13.8).
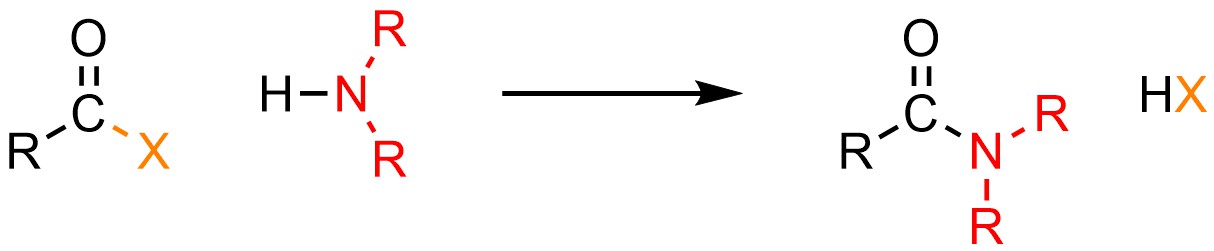
Scheme 13.8 – Generalized Reaction Equation for Addition-Elimination Converting Acid Halides to Amides.
The side product of this reaction is a strong acid (HX) instead of a salt (NaX). Because the amine is also a base, this has an unintended consequence: for every molecule of amine that undergoes the addition-elimination, another molecule of the amine is consumed in an acid-base reaction. If the reaction is performed without considering this the maximum theoretical yield is 50% (half of the amine is used for addition-elimination, half for acid-base; Scheme 13.9).

Scheme 13.9 – Generalized Reaction Equation for Addition-Elimination Converting Acid Halides to Amides Using One Equivalent of Amine.
There are two common approaches to improving this. Two equivalents of the amine can be added to the reaction (Scheme 13.10). This is the solution if the amine is simple/affordable. Alternatively, another non-nucleophilic base can be added to the reaction. The non-nucleophilic base used cannot be a hydride such as sodium hydride (NaH). Although they are non-nucleophilic hydrides can undergo other side reactions with carbonyl-containing functional groups. Instead, a sterically hindered/bulky tertiary amine is typically used. These amines have enough steric interactions that they cannot easily act as nucleophiles but remain bases. By far the most common amine for this purpose is diisopropylethylamine (DIPEA).
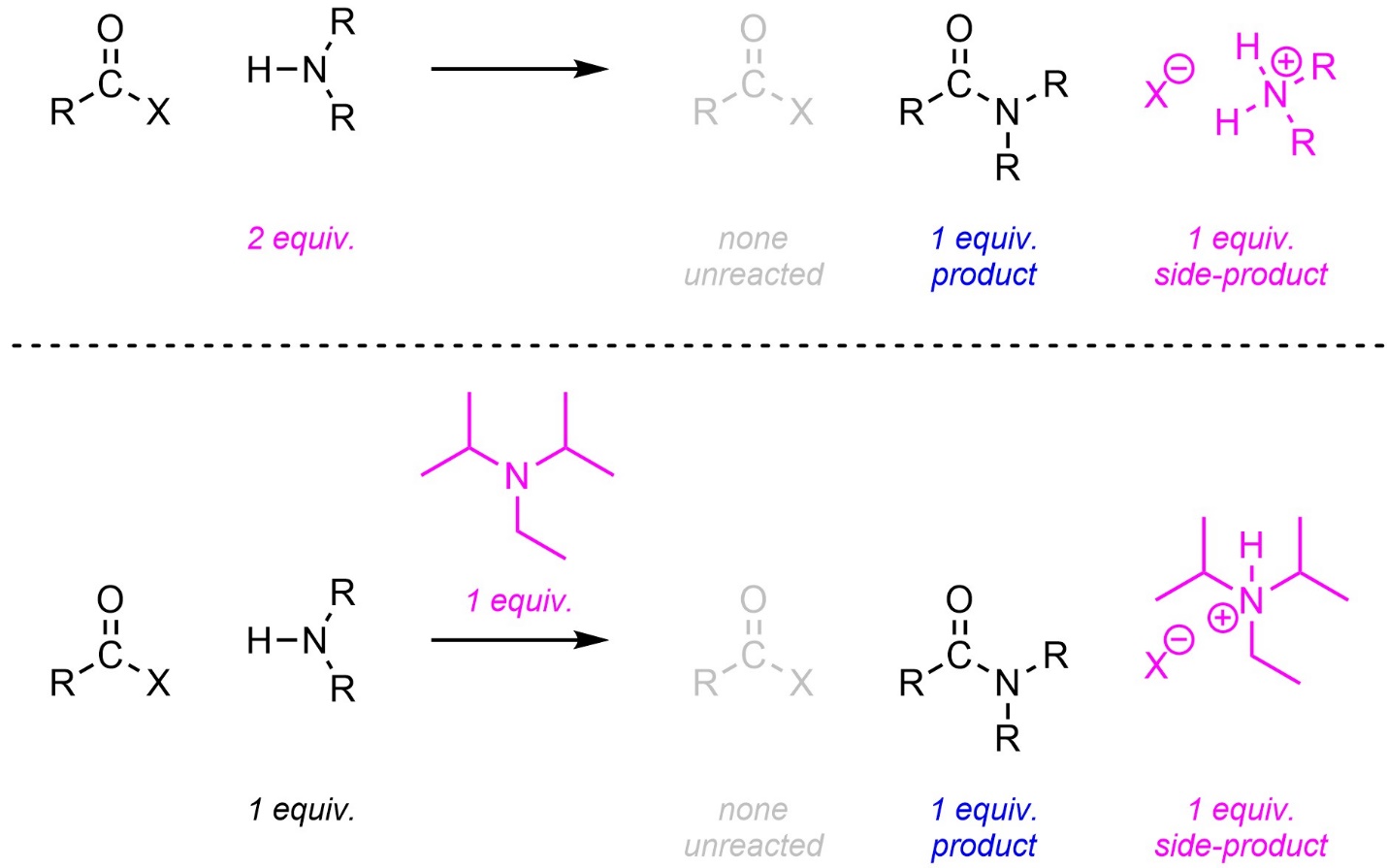
Scheme 13.10 – Generalized Reaction Equations for Addition-Elimination Converting Acid Halides to Amides Using Two Equivalents of Amine or A Second, Non-Nucleophilic, Base.
13.5.3.1. Mechanism
This reaction follows the standard two step mechanism for addition-elimination reactions (see Section 13.3) followed by a deprotonation (Scheme 13.11). The nucleophile (amine) attacks the electrophile (acid halide). This forms a new bond and adds a lone pair to the oxygen. Then that lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group (halide). Finally, a base (amine) removes a proton to generate the final product and a salt.
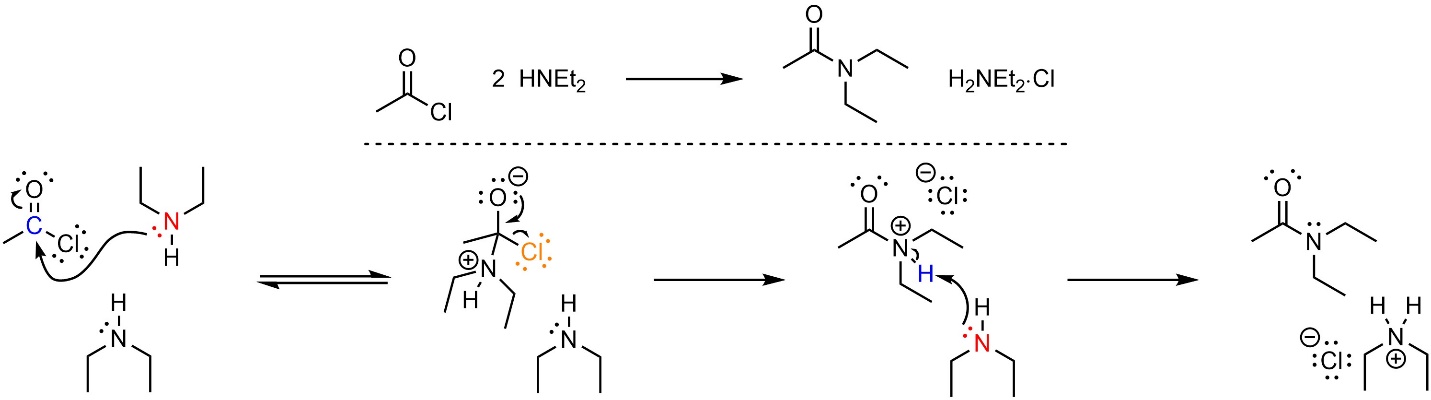
Scheme 13.11 – Reaction Mechanism for Addition-Elimination (Acylation) of Diethylamine with Ethanoyl Chloride Using Two Equivalents of Amine.

