4.6. Physical Properties of Enantiomers vs. Diastereomers
Enantiomers are physically and chemically identical (Table 4.1). Both enantiomers will have exactly the same boiling/melting point, exactly the same colour, undergo the exact same chemical reactions (at exactly the same rates), etc. Knowing the physical and chemical properties of one enantiomer tells you the physical and chemical properties of the other because they are identical.
Table 4.1 – Select Physical Properties of (S)-Alanine and (R)-Alanine.

There are two exceptions to this.
Enantiomers do not interact with other chiral molecules identically. This is generally true of all chiral objects. For example, your left and right hands interact with a ball (achiral) in exactly the same way. However, your left hand shakes hands properly with someone else’s left hand and improperly with someone else’s right hand. Conversely, its enantiomer (your right hand) shakes hands improperly with someone else’s left hand and properly with someone else’s right hand. They also interact with other chiral things (like right-handed scissors) differently.
This has important consequences for biology and chemical synthesis but is too complex a topic to explore in-depth.
Enantiomers do not interact with plane polarized light identically (see Section 4.7.). This has few implications or applications but is useful when determining which enantiomer (or mixture of enantiomers) exists in a sample.
Diastereomers are physically and chemically different (Figure 4.38). They will have different boiling/melting points, different colours, may undergo different chemical reactions (usually they undergo the same chemical reactions but at different rates), etc. It is important to remember that ‘different’ is a matter of degree; the physical or chemical properties of diastereomers might be coincidentally similar. Knowing the physical and chemical properties of one diastereomer tells you nothing about the physical and chemical properties of the others because they are different.
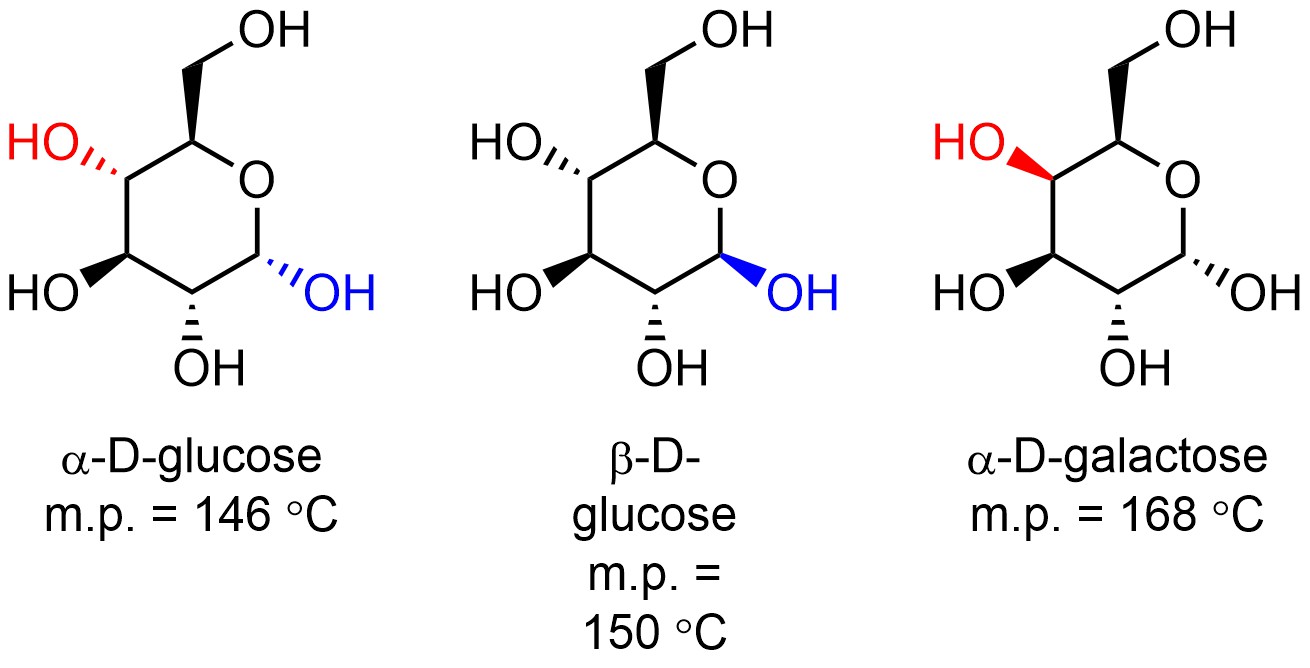
Figure 4.38 – Example of Diastereomers with Different Melting Points.
An in-depth exploration into the consequences of enantiomers interacting with other chiral molecules differently is beyond the scope of this text. However, it is very important that the gravity of this be conveyed. As such, an example of these consequences is typically discussed in introductory works.
Biological systems are full of chiral molecules. Proteins, enzymes, DNA, RNA, sugars, hormones, and more are all chiral compounds. In 1957 the drug thalidomide entered the market as a treatment for morning sickness (Figure 4.39).
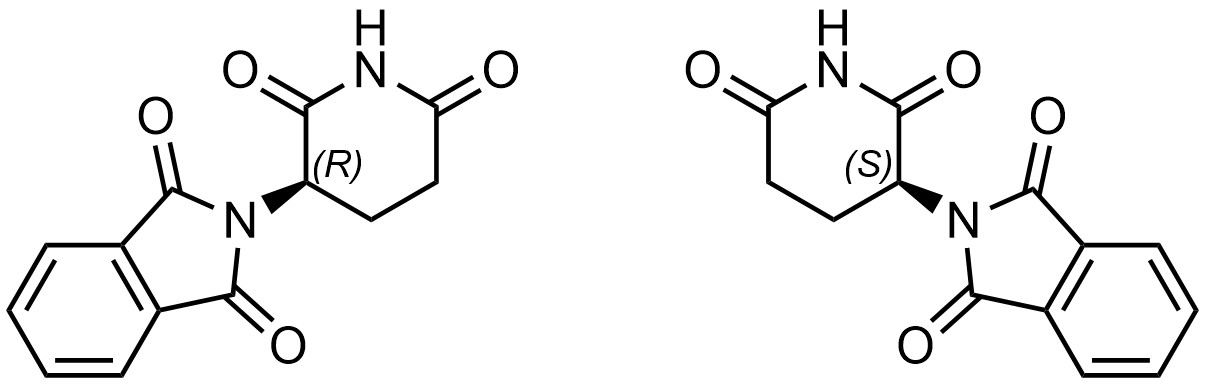
Thalidomide can be prepared as (functionally) a single enantiomer, though it can interconvert between the two isomers once in the body (how this occurs is not important). At the time, it was cheaper to produce the drug as a mixture of the two enantiomers. The interconversion and initial low stereochemical purity resulted in a problem.
(R)-Thalidomide treats nausea and has few side effects. However, (S)-thalidomide does not treat nausea and is teratogenic. That is, (S)-thalidomide interacts with DNA, a chiral compound, in a way that is different from (R)-thalidomide. More specifically, (S)-thalidomide intercalates with the base pairs of DNA, causing errors when making copies of the DNA. This results in mutations. The effect is especially pronounced in fetuses, which are undergoing rapid cell division (i.e. making many copies of DNA as the fetus grows).
With thalidomide marketed for morning sickness, the results were disastrous. By the time the drug was removed from the market thousands of infants had perished shortly after birth, and tens of thousands more were born with moderate to severe physical or cognitive abnormalities. The resulting backlash led to an extreme increase in drug regulation in many countries, though at great cost.

