11.5. Solving Problems Using Special Nucleophiles
In real life nucleophilic substitution reactions do not always proceed smoothly. Sometimes, it is necessary to adjust the nucleophile to avoid having other reactions competing with the desired one. These are sometimes referred to as side reactions and produce side products (products that are different from the one that was desired).
11.5.1. Using Acetates to Add OH
Recall that, generally, nucleophilicity and basicity may be considered equivalent: compounds that are strong nucleophiles are also strong bases. The only additional consideration at an introductory level is steric interactions, which affect nucleophilicity but not basicity.
Because most strong nucleophiles are also strong bases, nucleophilic substitution reactions may have issues with elimination reactions occurring as side reactions (see Chapter 12; Scheme 11.17).
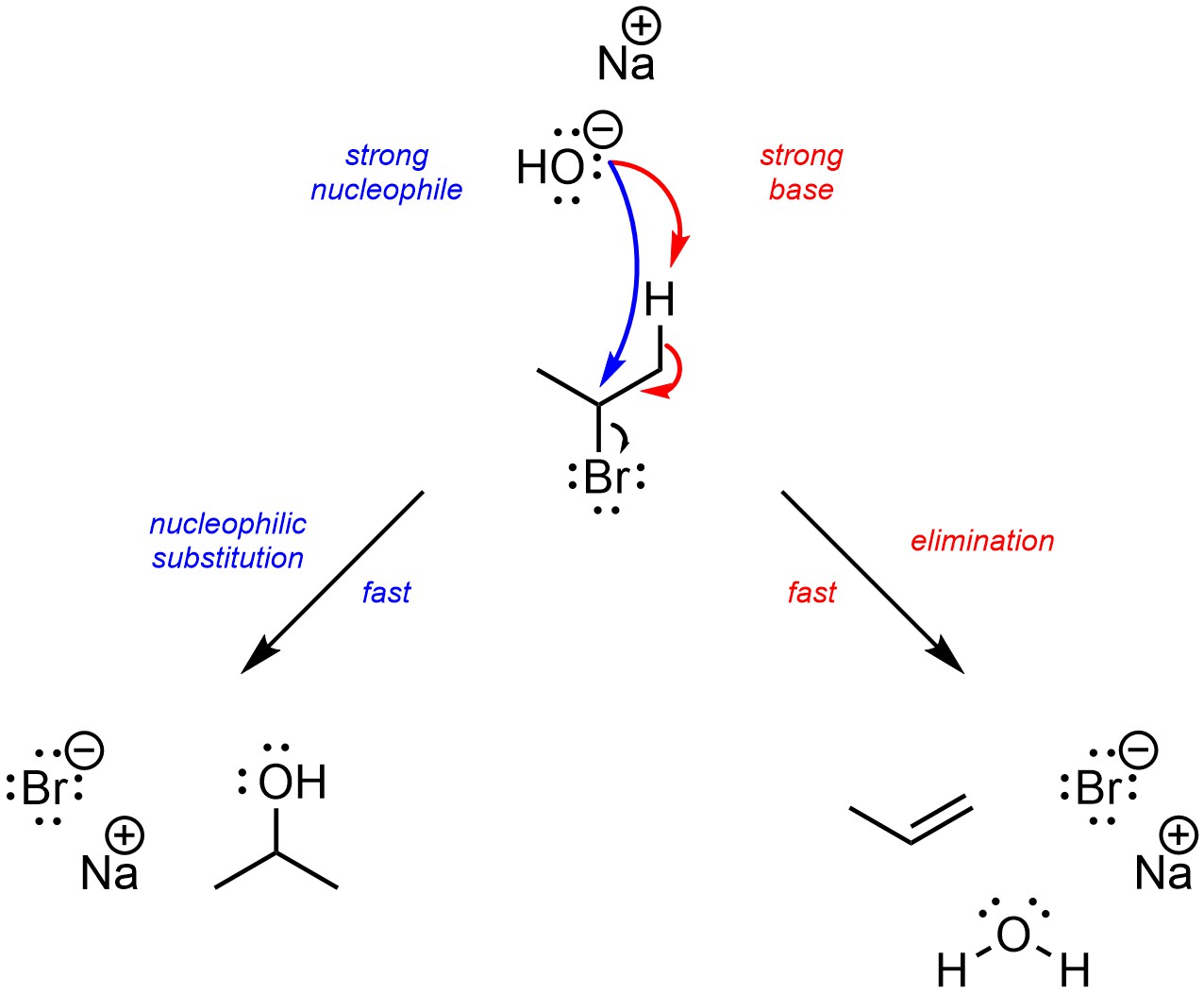
Scheme 11.17 – Example of Sodium Hydroxide, a Strong Nucleophile and Strong Base, Undergoing Two Competing Reactions with 2-Bromopropane.
This is particularly a problem for converting alkyl halides into alcohols using hydroxide as the nucleophile. Hydroxide is both a strong nucleophile and a strong base. Directly using it as a nucleophile may make the desired C-O bond, but also causes a side reaction to occur. Instead, a common solution is to use acetate (the conjugate base of acetic acid) as the nucleophile (Scheme 11.18). Acetate is only moderately nucleophilic, but it is a weak base. As a result, the nucleophilic substitution reaction is (much) faster than the side reaction, so more of the desired product is formed.
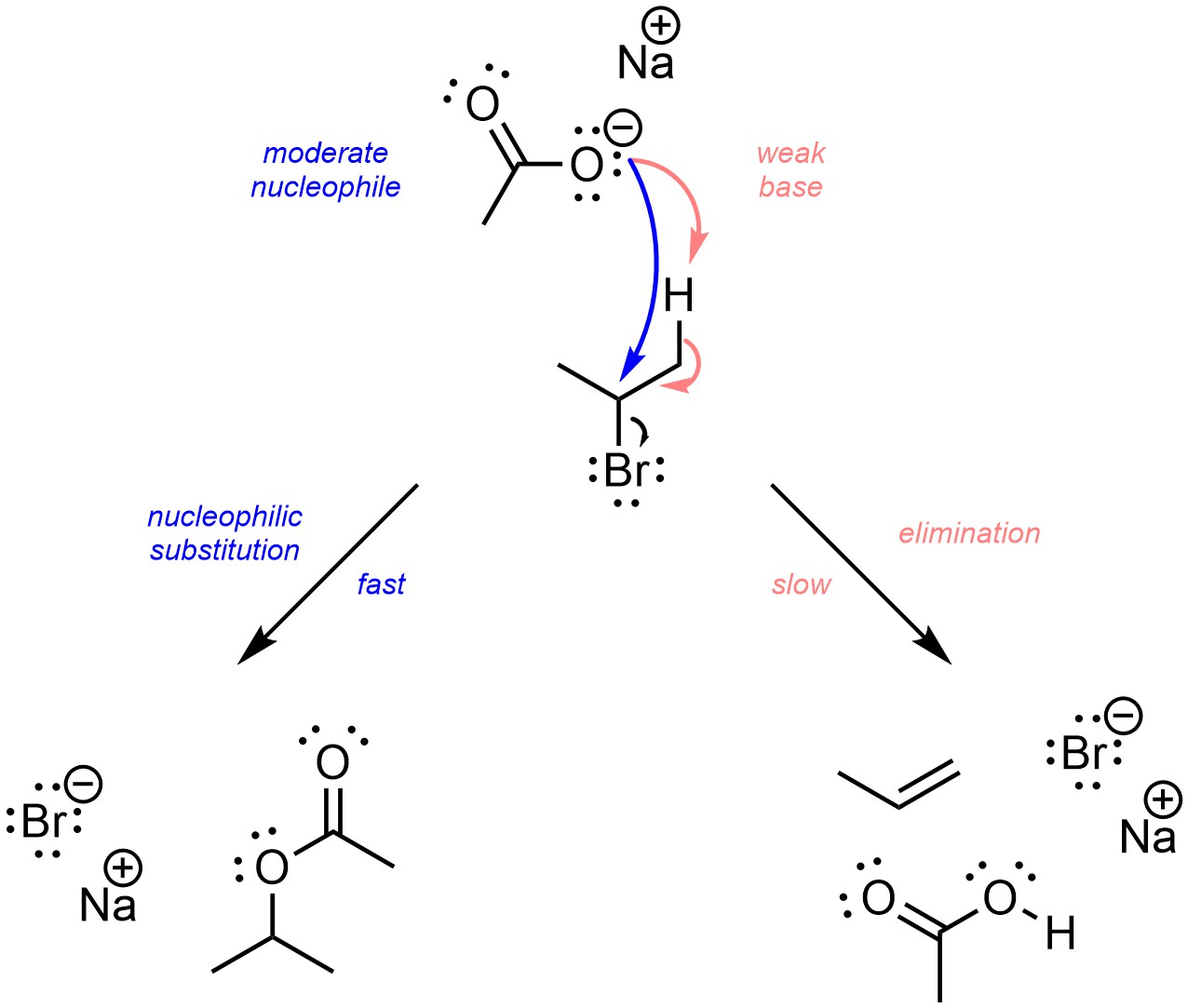
Scheme 11.18 – Example of Sodium Acetate, a Moderate Nucleophile and Weak Base, Selectively Undergoing One of Two Competing Reactions with 2-Bromopropane.
This forms an ester rather than the product that was desired, an alcohol. However, there are several simple reactions that can convert esters into alcohols (see Chapter 13). Although two reactions are needed to generate the alcohol, the overall yield of the desired product is usually much higher than if hydroxide were used directly.

