12.5. Reaction: Elimination of H-OH (Dehydration)
Two types of elimination reactions occur very frequently in synthesis. Dehydration, the elimination of water (H-OH), is a very common elimination reaction (Scheme 12.10). It is possible for this reaction to occur without catalysis, or with base catalysis. However, in the overwhelming majority of cases chemists prefer to use acid catalysis for dehydration reactions.
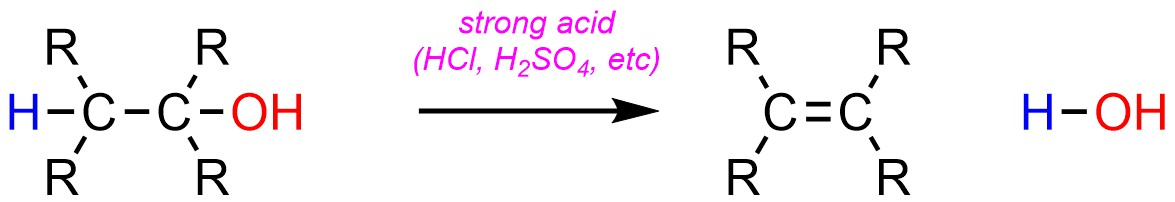
Scheme 12.10 – Generalized Reaction Equation for Acid-Catalyzed Elimination of H-OH to Form an Alkene.
12.5.1. Mechanism Using Acid Catalysis
Depending on the structure of the starting material this reaction may proceed through an E2 or E1 mechanism. For comparison to hydration (addition of H-OH across an alkene, see Section 8.6.1) an example following the E1 mechanism is shown. The mechanism for this reaction is the exact opposite order of steps as the addition of H-OH across an alkene (Scheme 12.11; see Scheme 8.9). The strong acid reacts with water to form hydronium (not shown). This is the active catalyst. First, the leaving group (alcohol) gets activated by the catalyst. This greatly increases its leaving group ability. The activated leaving group leaves, generating a carbocation and water. Finally, another equivalent of water removes a proton, generating the final product and regenerating the catalyst.
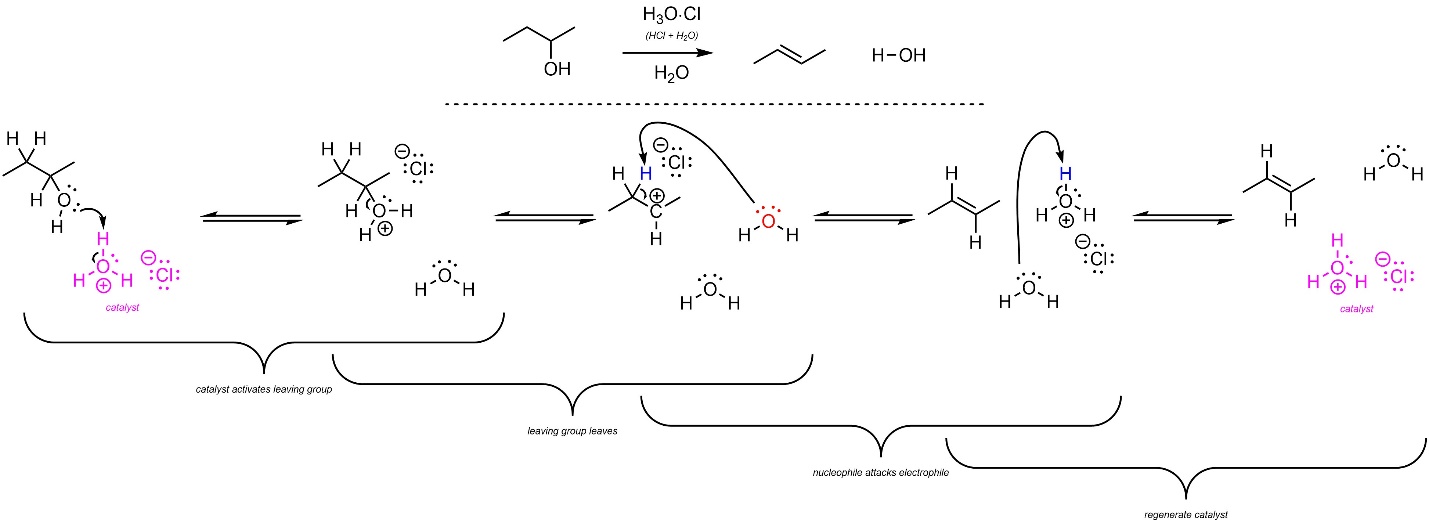
Scheme 12.11 – Reaction Mechanism for Acid-Catalyzed Elimination of H-OH to Form the Alkene of (E)-But-2-ene.
12.5.2. Controlling Equilibrium
Although the reaction equations imply that they are irreversible both hydration and dehydration are fully reversible and may be catalyzed by the same catalyst. As a result, the alkene and the alcohol are in equilibrium. It is possible to control the equilibrium using Le Chatelier’s principle. The strong acid catalysts used for these reactions, such as hydrochloric acid (HCl) or sulfuric acid (H2SO4), are dissolved in water (H2O). As a result, lowering the concentration of the acid catalyst also adds water to the reaction. Because water lies on one side of the equilibrium, changing the concentration of the acid catalyst can shift equilibrium to favour either the alkene or the alcohol (Scheme 12.12).
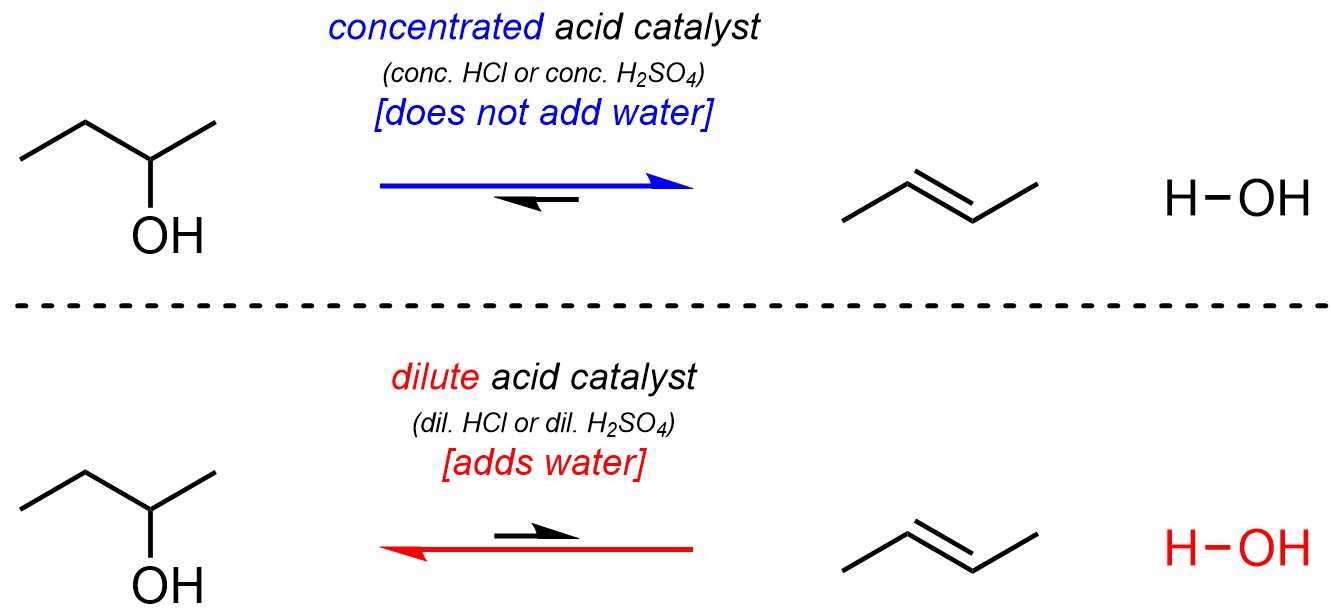
Scheme 12.12 – Controlling Equilibrium in Hydration/Dehydration Reactions.

