9.4. How to Classify Compounds as Aromatic, Anti-Aromatic, and Non-Aromatic
A compound can be non-aromatic by breaking any of the four requirements of aromaticity. Conversely, the first three requirements must be met to be eligibly classed as either aromatic or anti-aromatic. Then, if the number of electrons in the π system of the ring satisfies Hückel’s Rule [4n+2] it is aromatic, and if it does not satisfy the rule [4n] it is anti-aromatic. With practice recognizing most aromatic systems becomes streamlined, though complex examples may still be challenging.
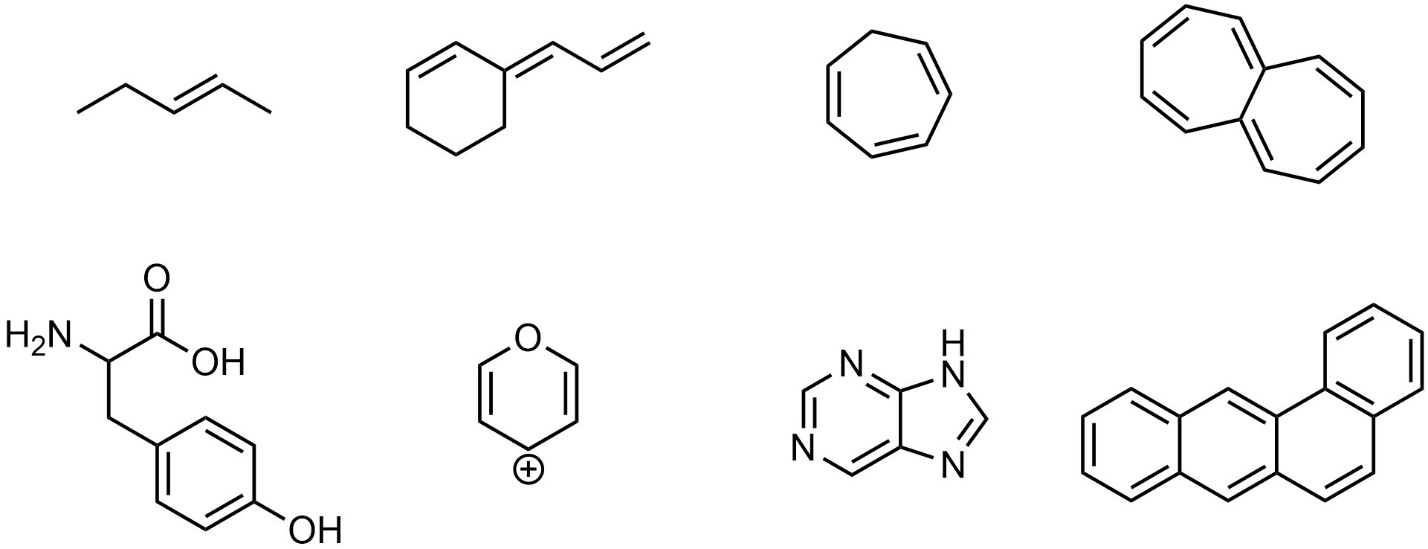
- Is there at least one cyclic structure (ring)? If no, it must be non-aromatic.
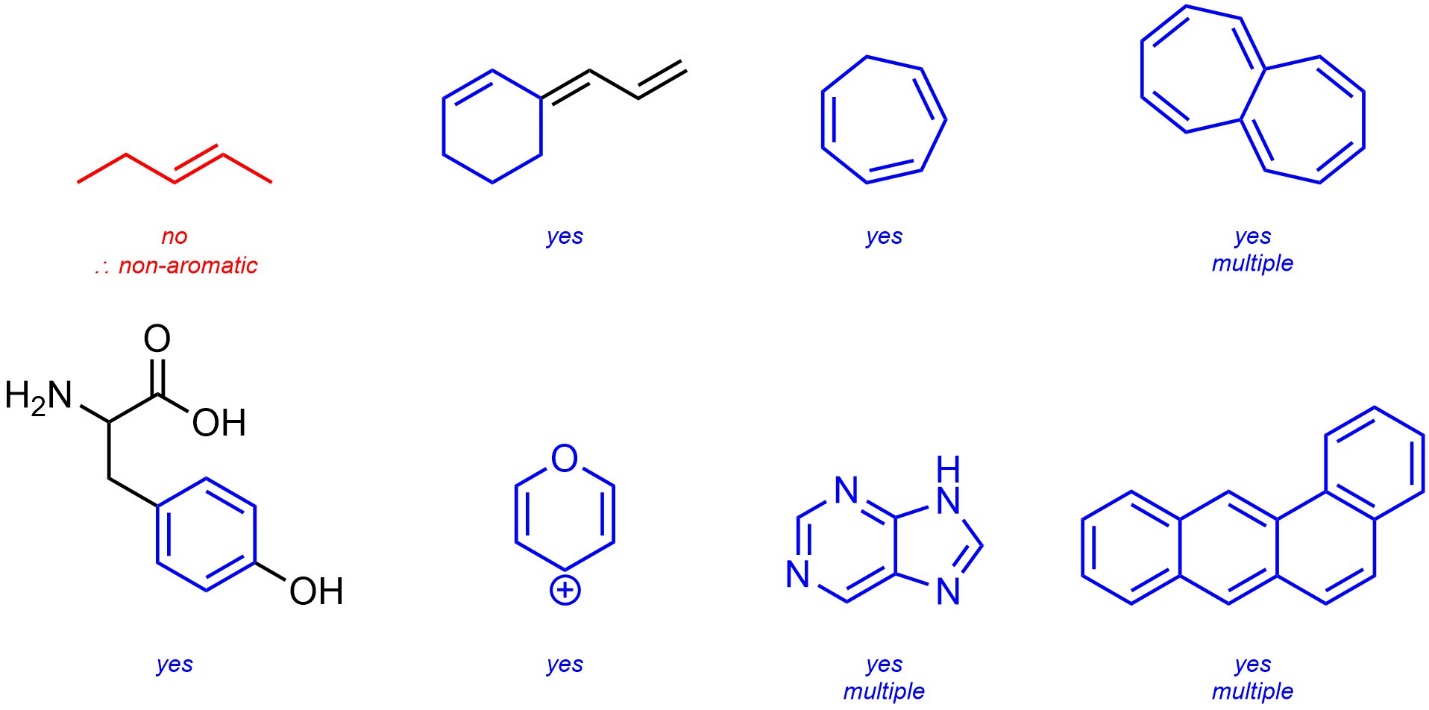
- Is there potentially a non-orthogonal p orbital on all atoms in the ring? When it is not obvious, draw in all lone pairs and at least one additional resonance structure (to assign hybridization). If no, it must be non-aromatic.
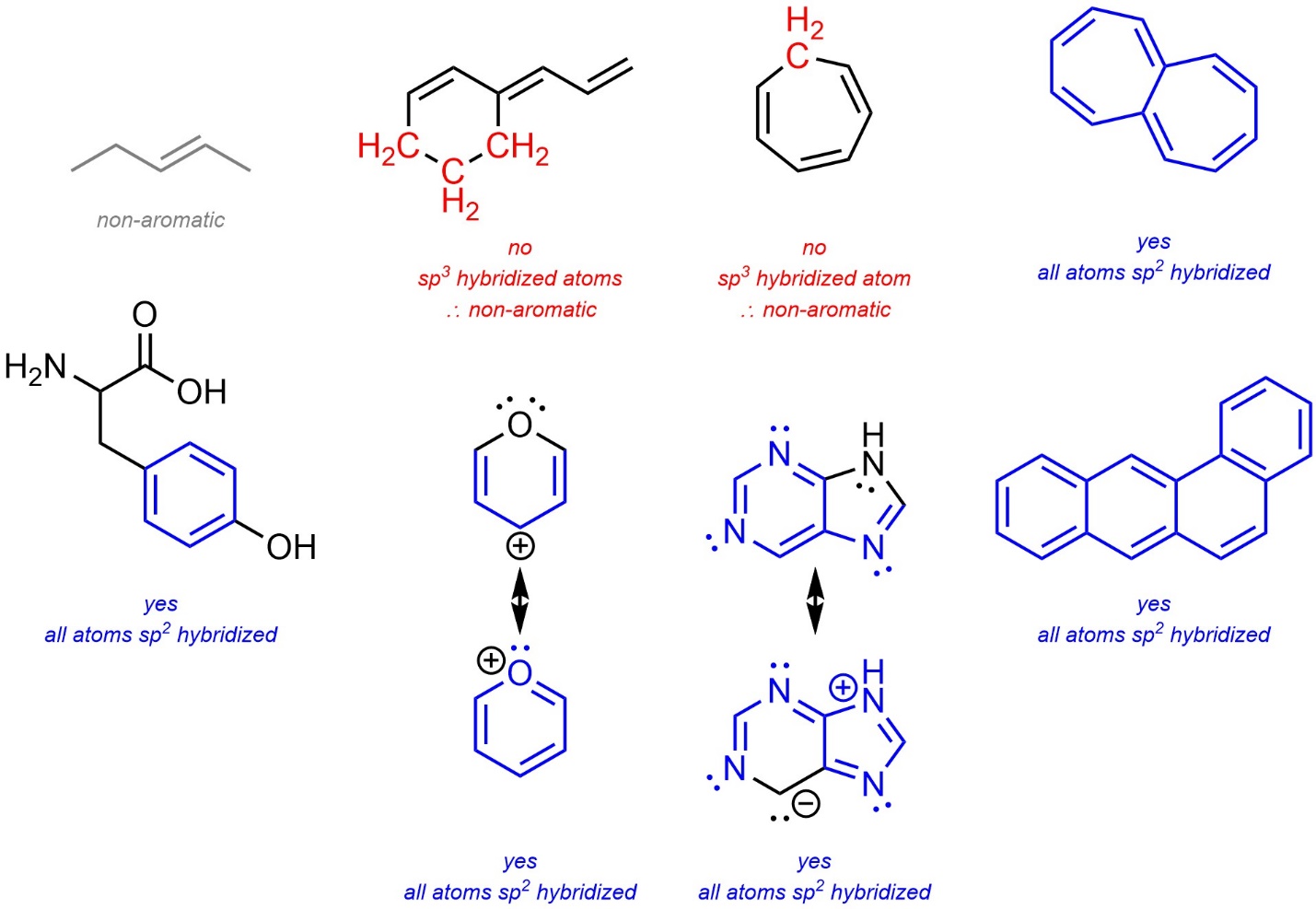
- If the number of π electrons in the ring(s) satisfies Hückel’s Rule [4n+2] it is potentially aromatic. If the number of π electrons in the ring(s) does not satisfy Hückel’s Rule [4n] it is potentially anti-aromatic. Remember that a single ring or subsection in a fused system may be (anti-)aromatic, or a part of a molecule may be (anti-)aromatic. As a result, this step can be complicated in large systems. Identifying the largest possible system that can be aromatic is a good approach for these cases. Remember that if part of a molecule can be aromatic, it will; if part of a molecule can avoid being anti-aromatic, it will.
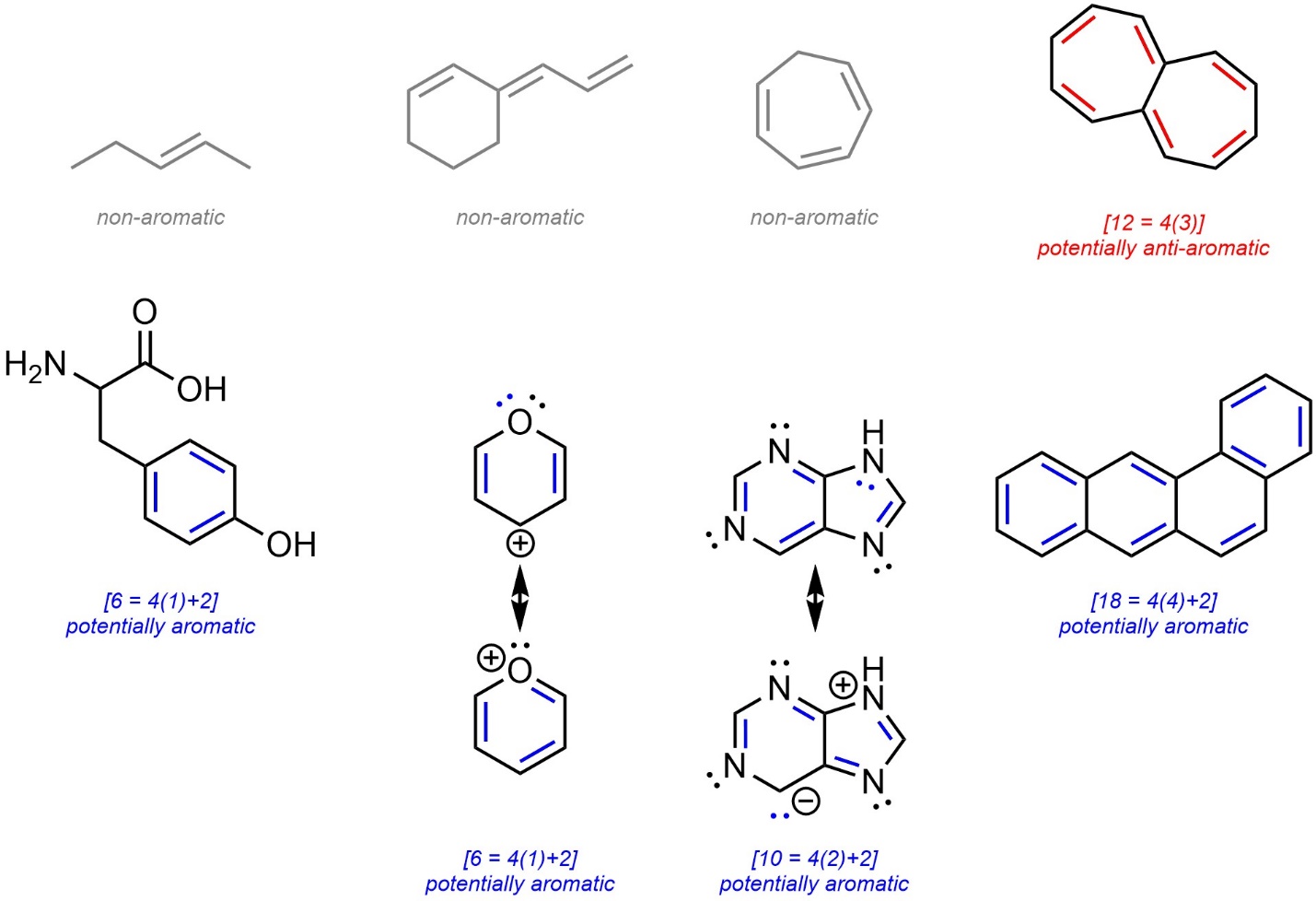
- If the ring system is potentially aromatic, check if it is possible for it to be planar. If it can, it is aromatic. If it cannot, it is non-aromatic. If the ring system is potentially anti-aromatic, check if it is possible to avoid being planar. If it can, it is non-aromatic. If it cannot, it is anti-aromatic.
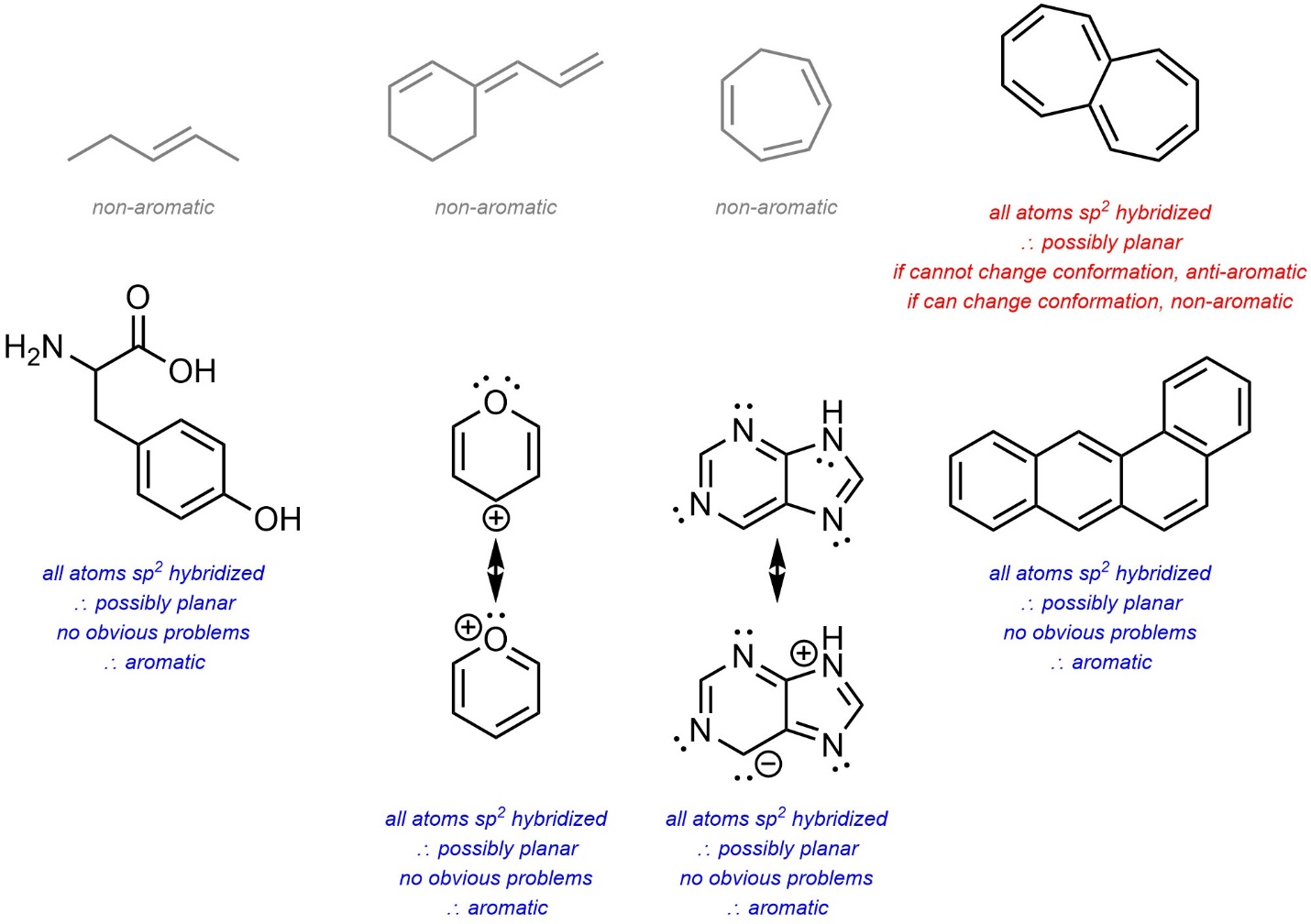
- Assign as possible.
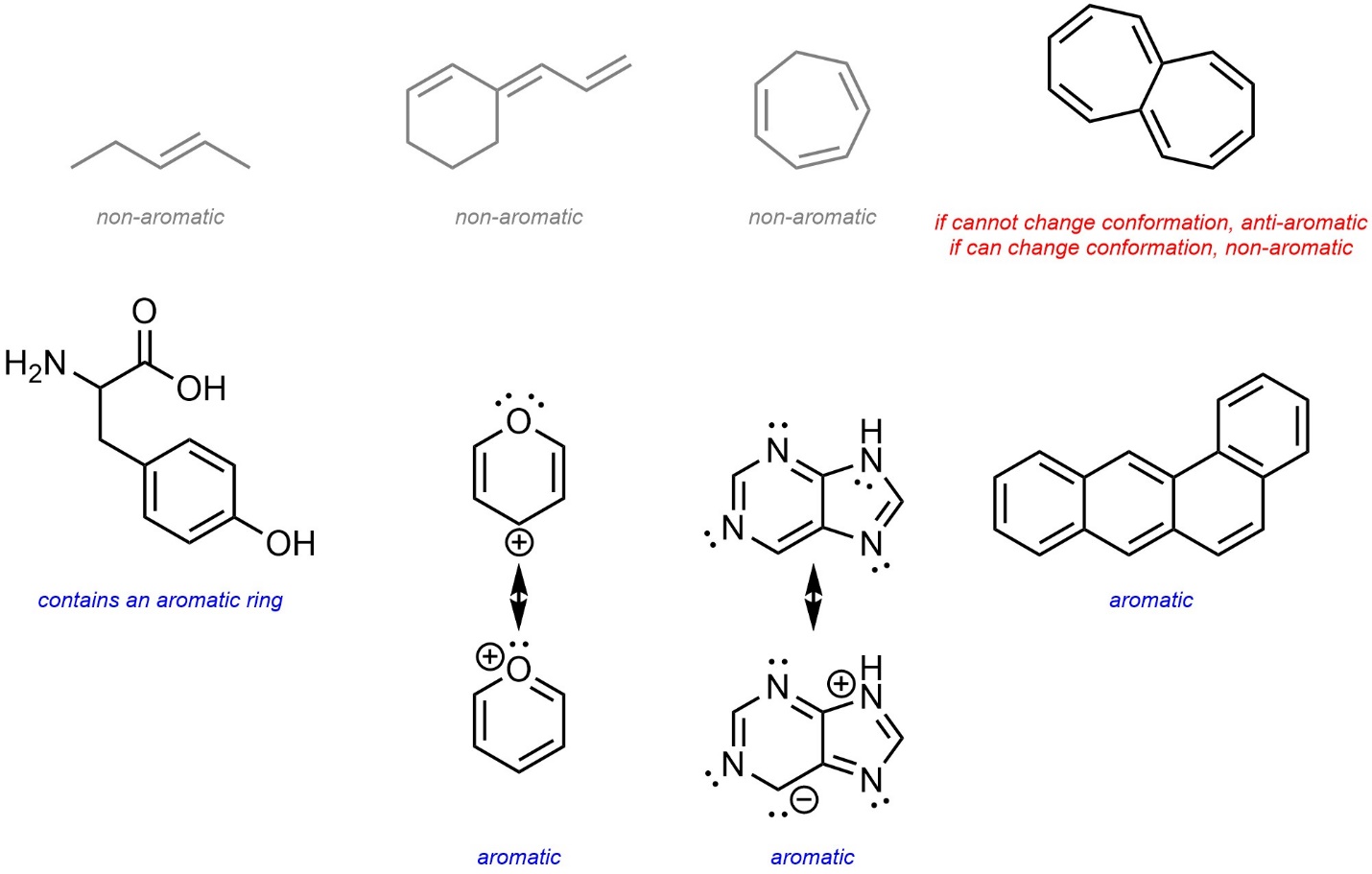
Ambiguity is often avoided by specific wording of questions. For example, “Is the planar conformation of this molecule aromatic, anti-aromatic, or non-aromatic?” has a definitive answer.