13.3. Mechanism, Reaction Coordinate, and Rate-Determining Step
Most addition-elimination reactions follow a simple two-step mechanism (Scheme 13.3). The nucleophile attacks the electrophile (carbonyl). This forms a new bond and adds a lone pair to the oxygen. Then that lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group. Depending on the specific reaction that is occurring there may be additional steps before or after these. For example, the nucleophile, electrophile, and/or leaving group may be activated first, or there may be a protonation or deprotonation afterwards.
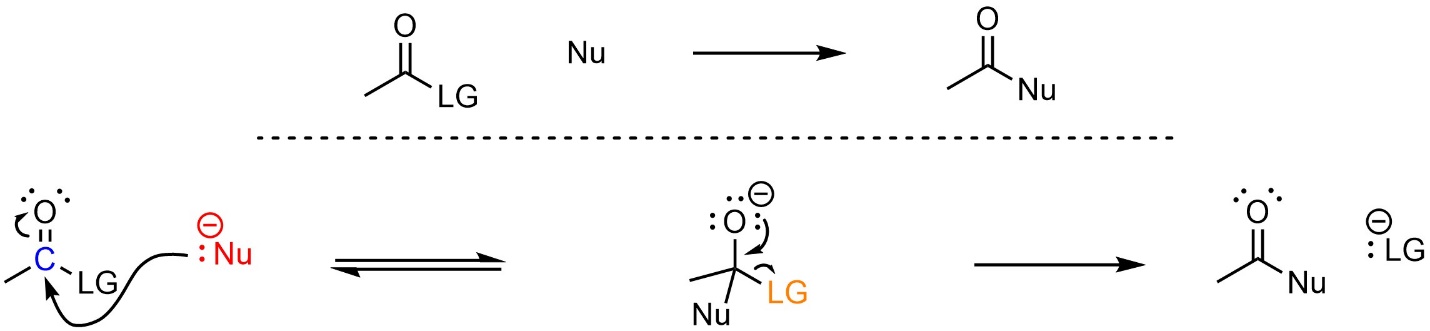
Scheme 13.3 – Generalized Reaction Mechanism for Addition-Elimination Reactions.
The first step, nucleophilic addition to the carbonyl, requires a moderate amount of energy and is usually the rate-determining step (Figure 13.1). However, depending on the strength of the nucleophile and the leaving group ability of the heteroatom in the intermediate the second step may be rate-determining.
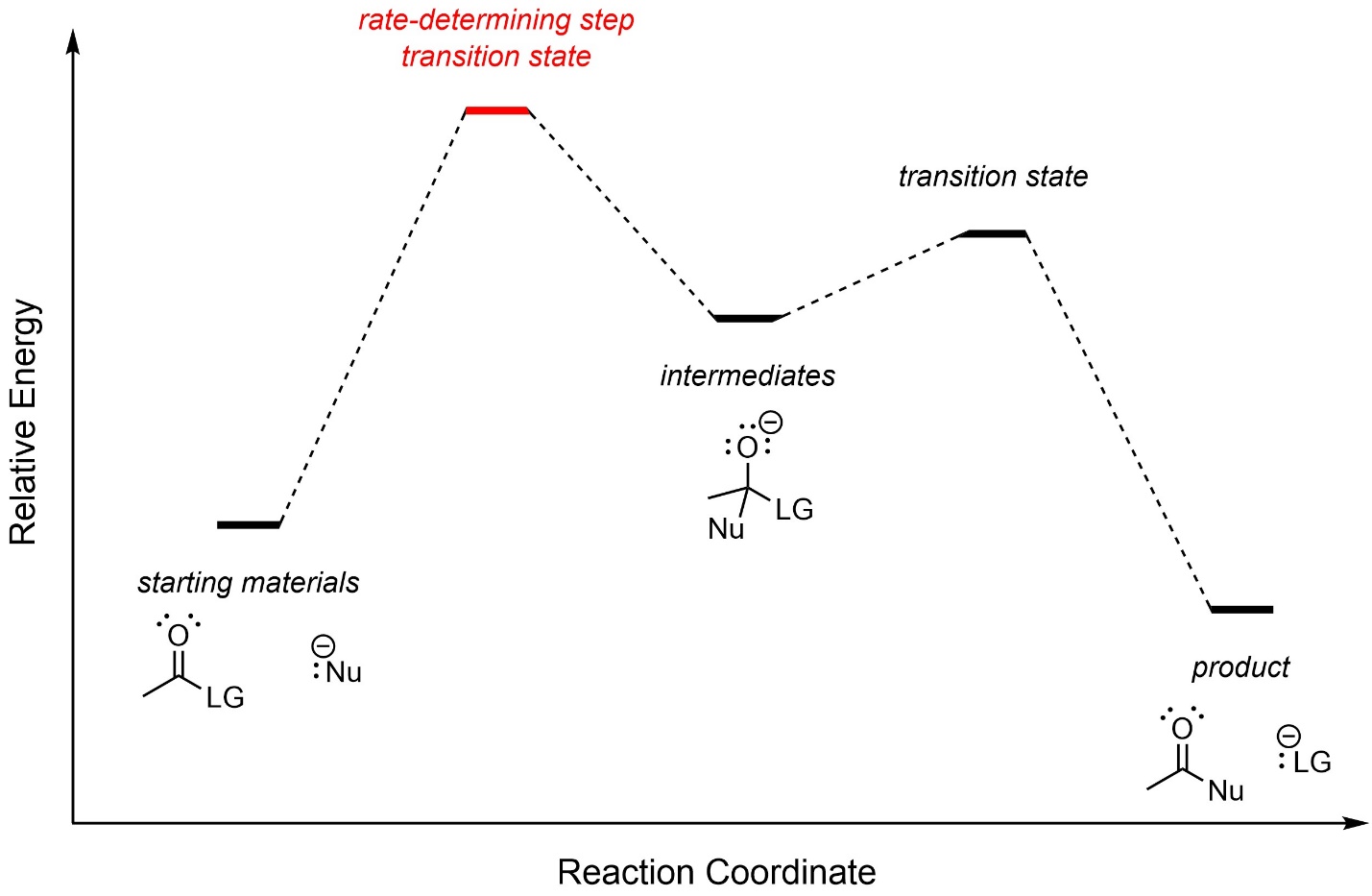
Figure 13.1 – Generalized Reaction Coordinate for Addition-Elimination Reactions.

