Chapter 5 Practice Problems – Answers
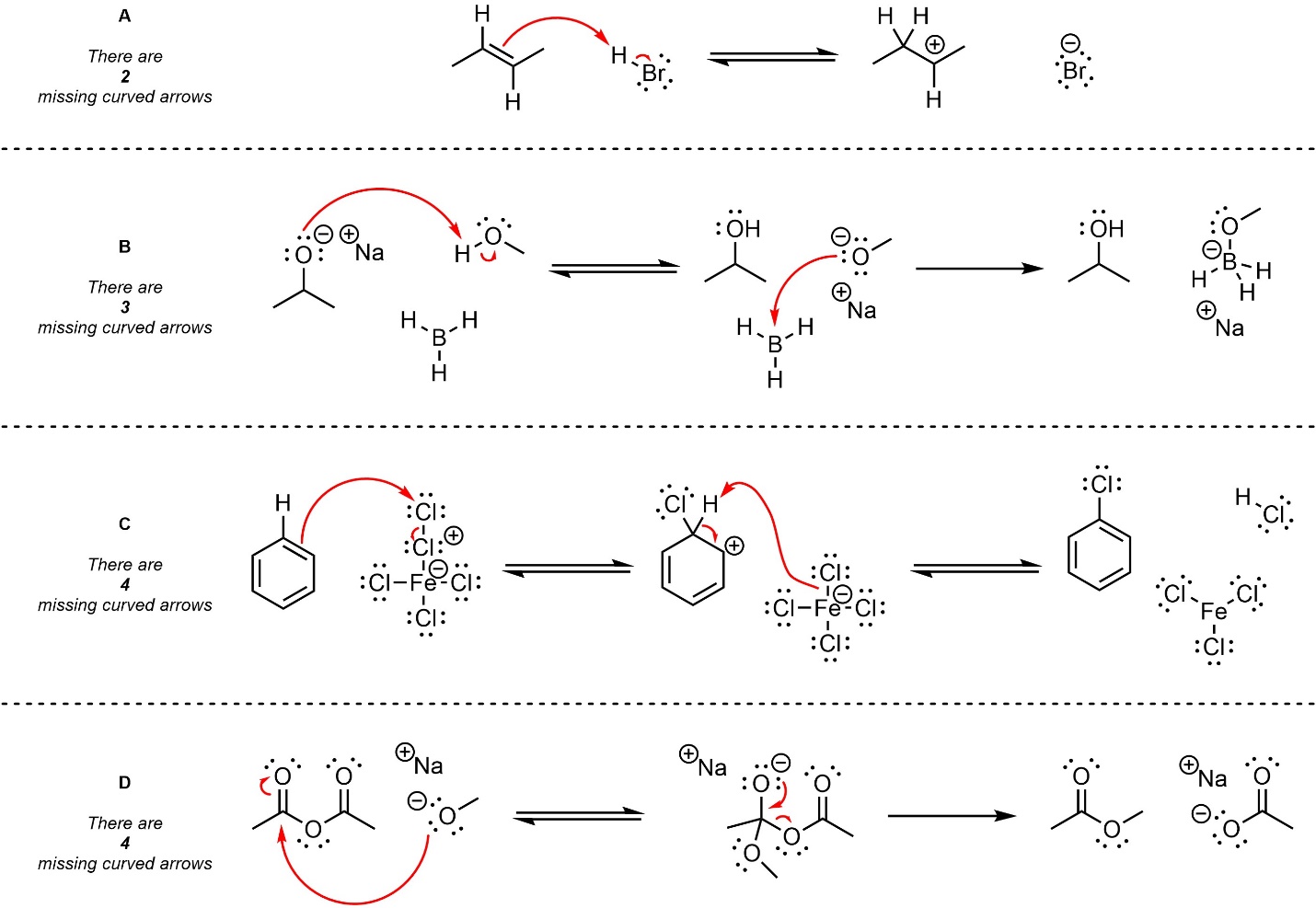
Q5.1: Below are several steps from mechanisms of hypothetical chemical reactions. The only parts missing are curved arrows representing electron flow. Add the missing curved arrows. For convenience the number of missing arrows from each example is given.
Paying attention to which bonds/lone pairs are new will help.
Sometimes it is helpful to circle or label atoms in each step
to see where they come from or go to.
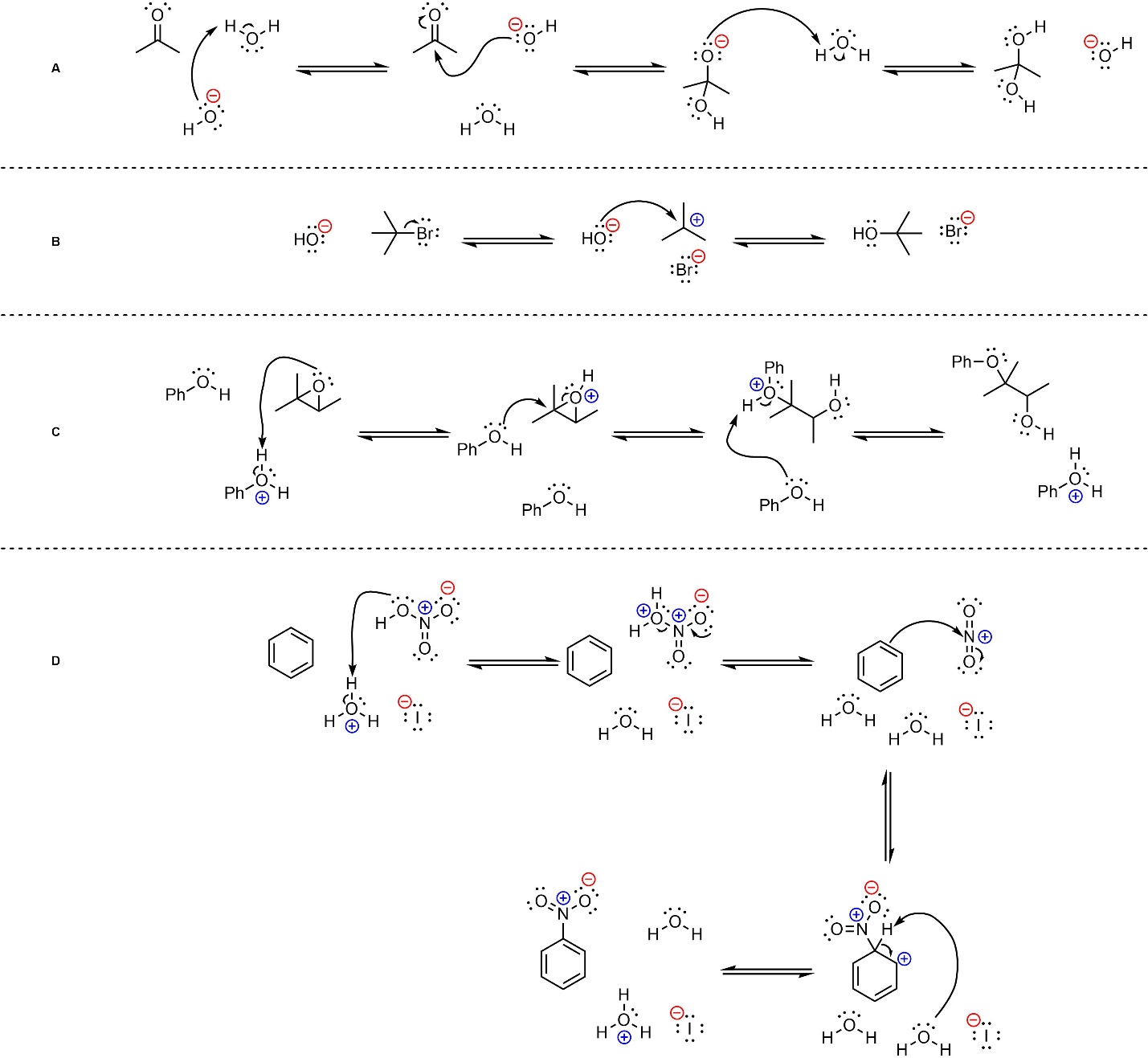
Q5.2: Below are mechanisms of hypothetical chemical reactions. The only parts missing are formal charges. Add the missing formal charges. Some questions are also missing a counterion (i.e. they will not be overall neutral at each step). Do not worry about the missing counterions, they will not affect the mechanism.
Carbocations may be challenging.
Pay attention to how many implied H’s there are before/after a step.
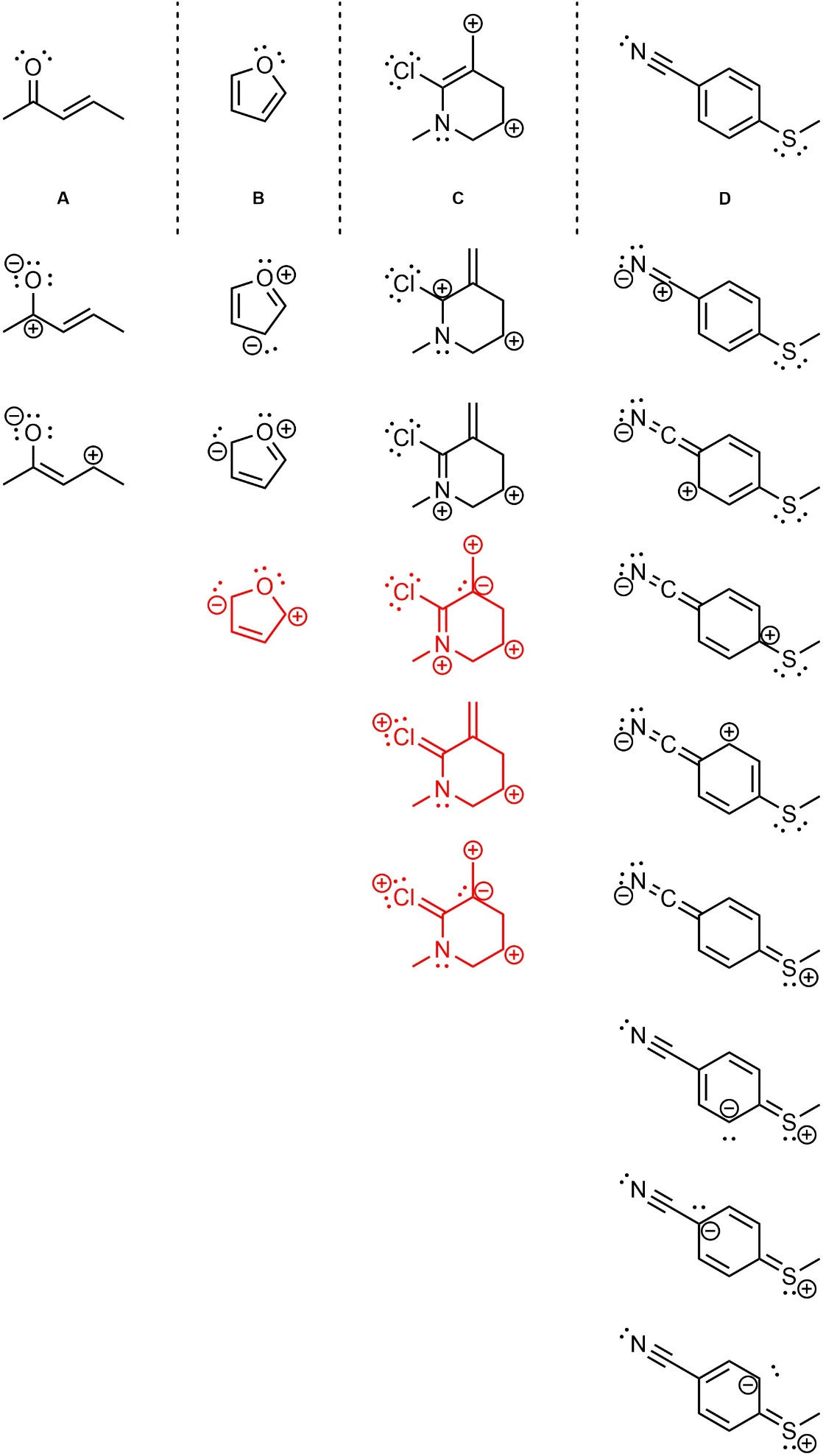
Q5.3: For each of the following, draw two additional significant resonance structures. Although it is a significant contributor, for D simply moving the electrons around the aromatic ring does not count (too easy).
Note: Adding all lone pairs and/or showing curved arrows is not required but will greatly assist in answering the question.
All valid answers are given.
Answers in red are theoretically possible but do not contribute significantly.
(they would normally be marked as incorrect or given small part marks on an exam)
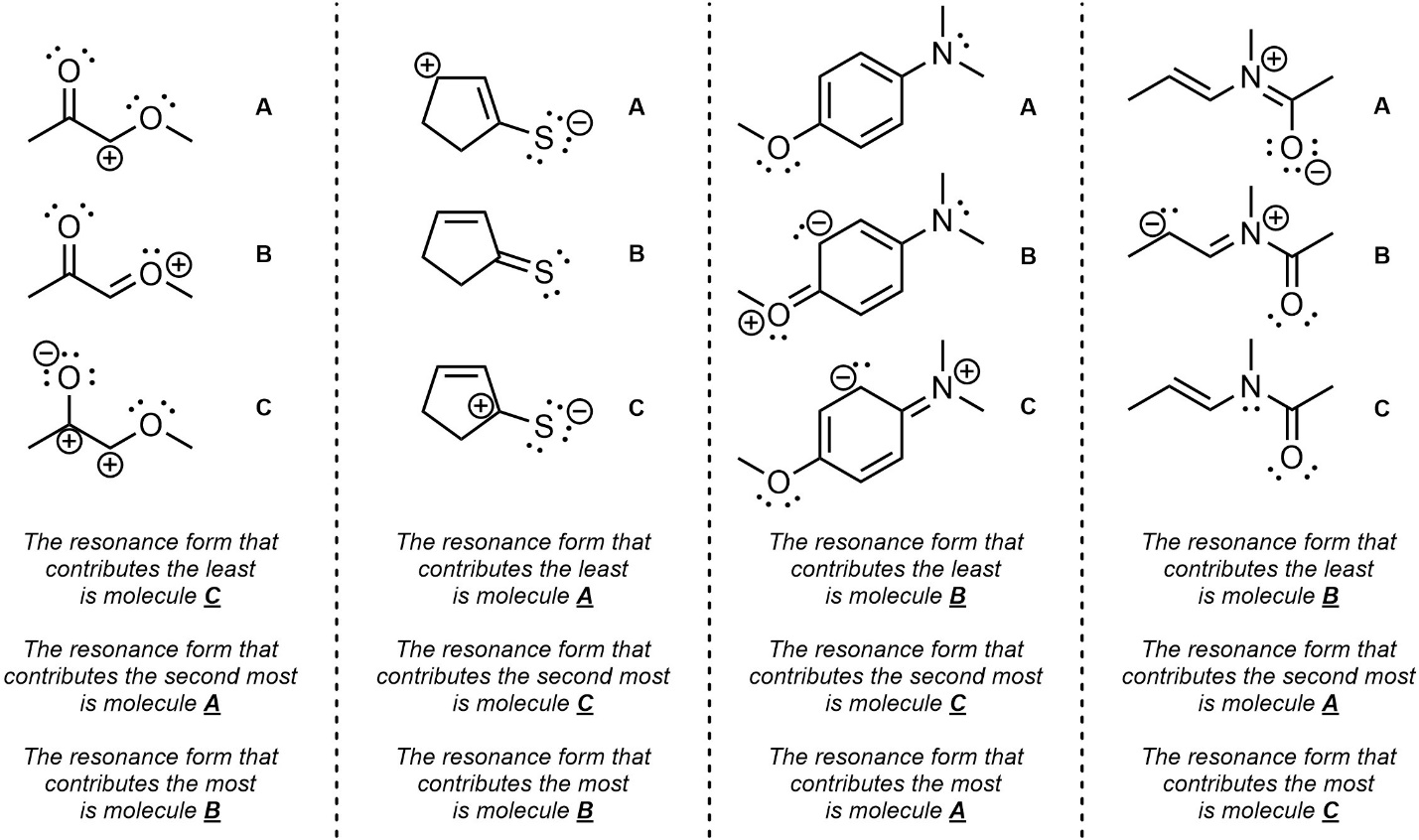
Q5.4: Rank each set of resonance forms in order of increasing contribution (lowest to highest).
Follow the considerations in order of importance.
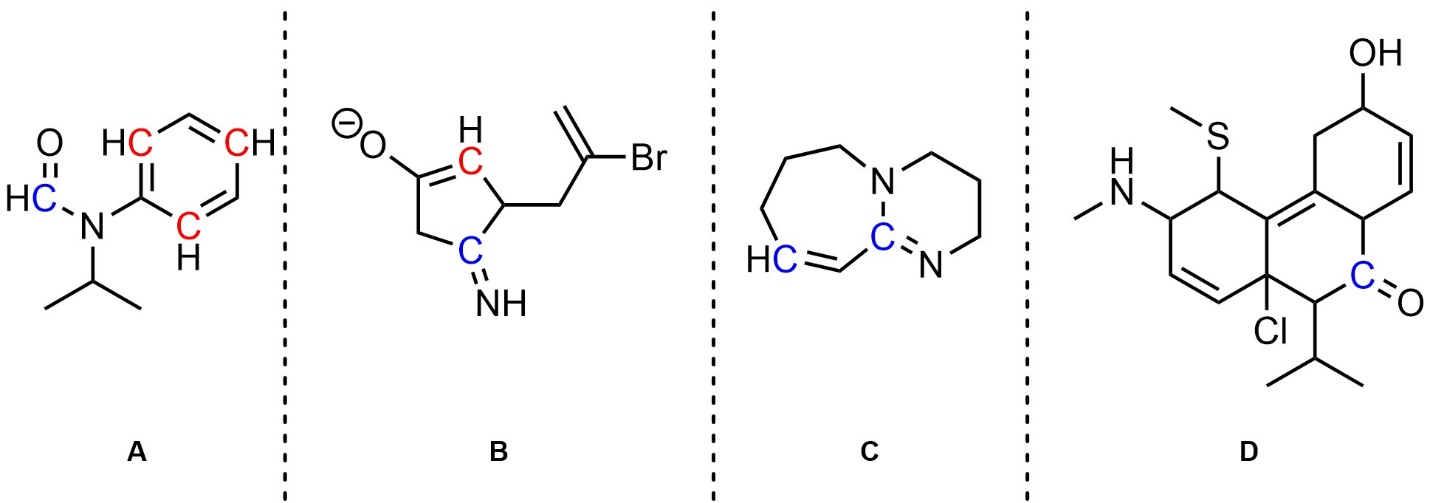
Q5.5: In the resonance forms provided all carbon atoms are neutral. However, in other significant resonance forms some are charged (cationic/anionic). Indicate (circle, draw an arrow to, etc.) which carbon atoms are cationic/anionic in other resonance forms. Differentiate (colour, label, etc.) whether they are cationic or anionic in the other forms.
Note: Drawing additional resonance forms is not required but may assist in answering the question.
Blue carbons are cationic in other significant resonance forms.
Red carbons are anionic in other significant resonance forms.
Q5.6: The text below is a procedure for a hypothetical multi-step reaction. Condense the information into a (relatively) simple reaction scheme using “over-the-arrow” notation. For the purposes of practice include all commonly shown species and conditions (e.g. Figure 5.4). It is recommended to use discrete steps, but you may use implied (numbered) steps if you choose. The structures of named compounds and intermediates are included but not all are required for the reaction scheme.
Note: The exact reactions occurring are not important. These compounds are intentionally chosen to be challenging, using names and structures not familiar to the reader to encourage careful consideration of the role of each compound.
Pyrrolidinone (1.00 equiv.) was added to a round bottom flask. The vessel was fitted with a septum, purged under vacuum three times, and placed under inert (argon) atmosphere. Dichloromethane (CH2Cl2 (0.2 M)) and trimethyloxonium tetrafluoroborate (1.00 equiv.) was added. The mixture was then stirred for 24 h at room temperature (23 °C) under the argon atmosphere. Pentafluorophenyl hydrazine (1.00 equiv.) was added in one portion and stirring continued for 48 h at 0 °C. The solvent was evaporated. The resulting oil was suspended in pyridine (0.3 M) open to air. Thiocarbonyldiimidazole (1.1 equiv.) was added. The mixture was then stirred for 12 h at 35 °C.
Analyze each sentence for the relevant information (see below).
Context is often important.
There are multiple correct ways of displaying the information.
Example:
In the answer key below Step 1, Step 2, and Step 3 use
three different ways of including the reagents’ information.
Which way(s) you used does not matter.
Other style changes like this are fine as long as all of the information is included.
Some critical thinking may be required.
Example:
The solvent and concentration in step 2 are not specified.
They are probably the same as the previous step (nothing changed).
Some information is included in the procedure but not the scheme.
Including it is not “incorrect” as long as there are no sentences.
Example:
Writing “Argon”/“Ar”/“Argon atmosphere” (etc.)
in the arrow notation would be fine.
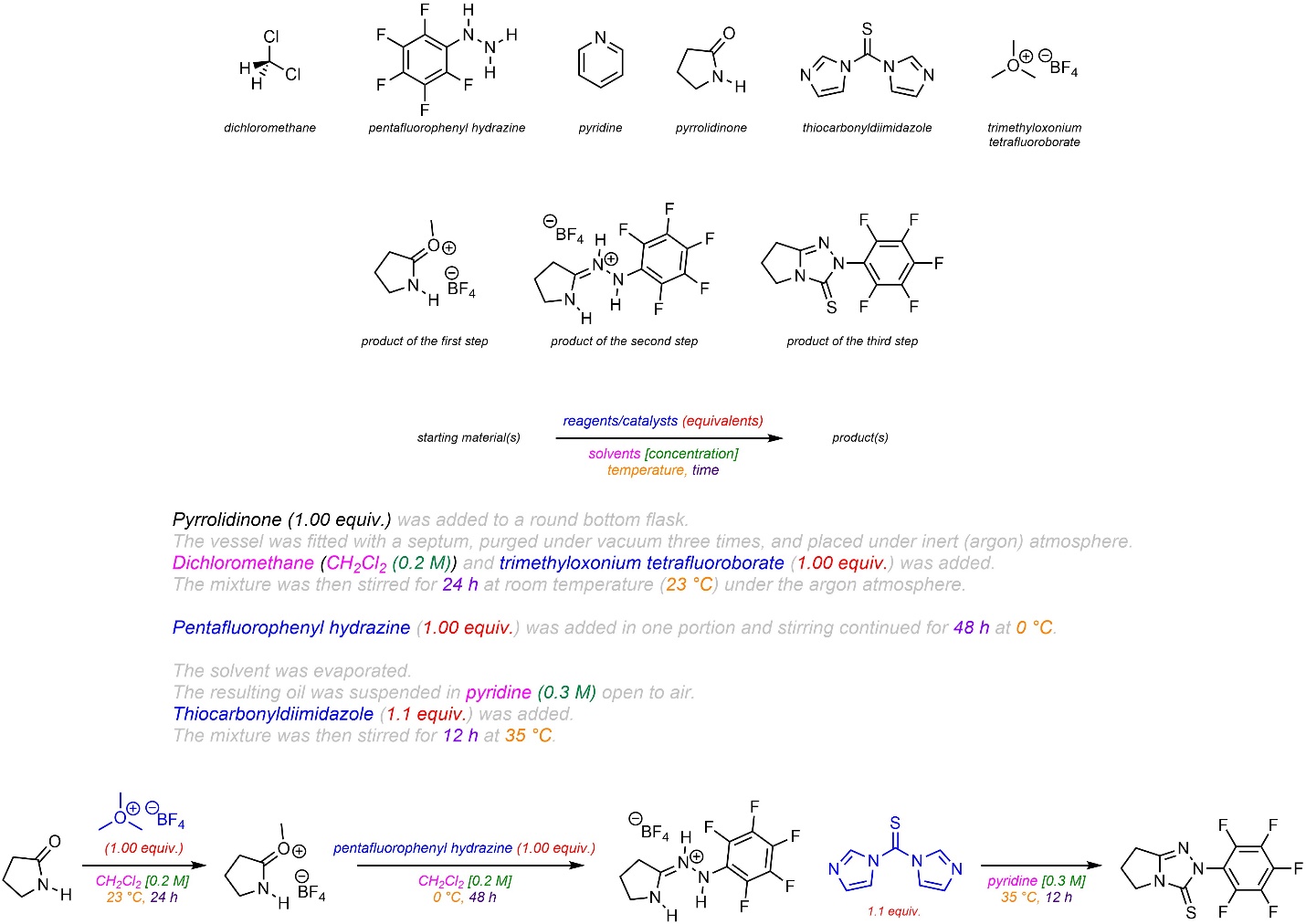
Colour-coding is added for clarity but is not required.
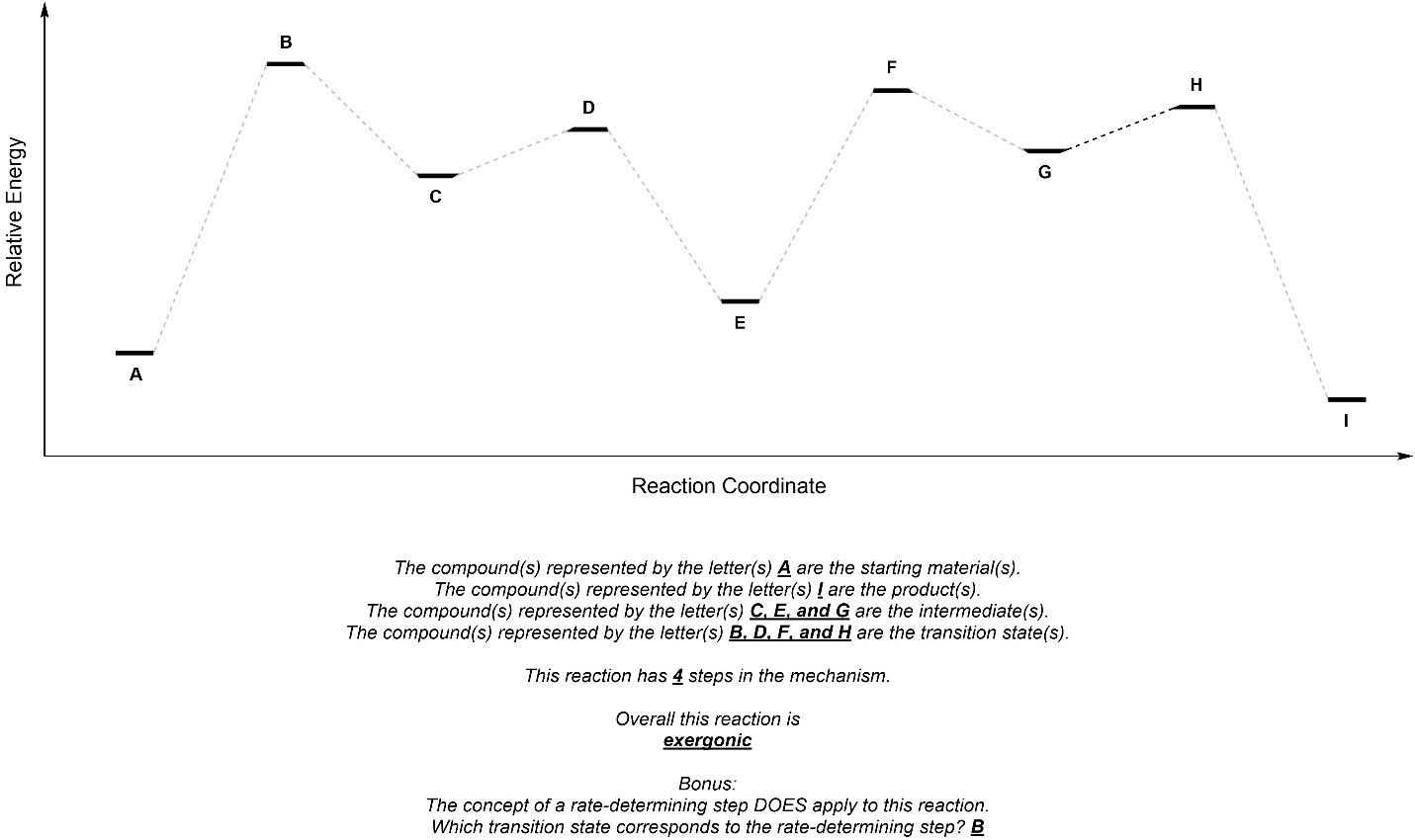
Q5.7: Fill in the blanks using the reaction coordinate below.