13.8. Reductions of Acyl Compounds Using Hydrides
Recall that the smallest potential nucleophile is hydride (H–; see Section 7.3.2). If nucleophilic hydride is desired a hydride source, where the hydride(s) is/are attached to a metalloid such as boron or aluminum, must be used. Depending on which metalloid is chosen there can be a large difference in nucleophilicity. For example, the hydrides of aluminum hydride (AlH4–) are much more nucleophilic than those of borohydride (BH4–).
Hydrides can react as nucleophiles with electrophilic carbonyls. During these reactions they may deliver multiple equivalents of hydride. For example, aluminum hydride (AlH4–) and borohydride (BH4–) are both able to participate in four hydride reductions/reactions each. Finally, recall that these reactions must be quenched using a strong acid (or strong base) to break the O-B or O-Al bonds and generate the final product. The overall reactions are often depicted as two steps to indicate that this is required.
Hydrides can attack carbonyl-containing functional groups other than aldehydes and ketones. This follows the standard mechanism and pattern for addition-elimination reactions (see Sections 13.2 and 13.3) except when the electrophile is an amide (see Section 13.8.4). After the addition-elimination reaction the product is a new carbonyl-containing functional group (Scheme 13.32). Another hydride may attack this product. This follows the standard mechanism and pattern for nucleophilic attacks of hydrides on carbonyls (see Section 7.3.2).
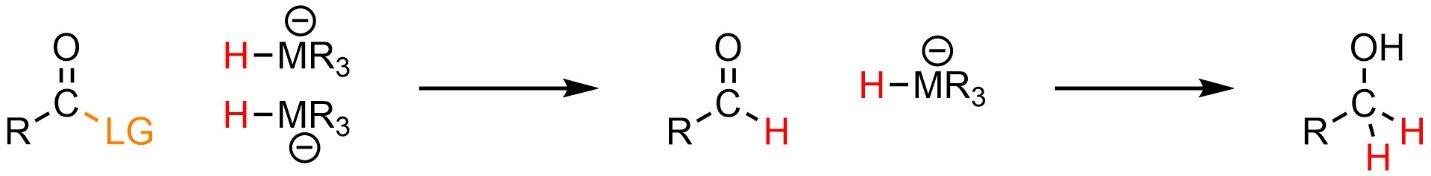
Scheme 13.32 – Generalized Reaction Equation for Addition-Elimination Followed by Addition When Using a Nucleophilic Hydride Source.
13.8.1. NaBH3CN vs. NaBH4 vs. LiAlH4
Sodium borohydride (NaBH4) and lithium aluminum hydride (LAH; LiAlH4) are the two most common hydride sources. There is a significant difference in nucleophilicity of the hydrides between these compounds. Other hydride sources are possible and typically have a tailored amount of nucleophilicity. For example, sodium cyanoborohydride (NaBH3CN) was designed to make the hydrides in it significantly less nucleophilic by exchanging one of the hydride groups for an electron-withdrawing group.
Structure directly affects chemical reactivity. The structural differences between the hydride sources affects their nucleophilicity; they have different chemical reactivities (Figure 13.7). Sodium cyanoborohydride is, relative to the others, weak as a nucleophile. As a result, it can only react with very strong electrophiles. Sodium borohydride is strong enough to attack more functional groups, while lithium aluminum hydride is able to attack all of the common carbonyl-containing functional groups.
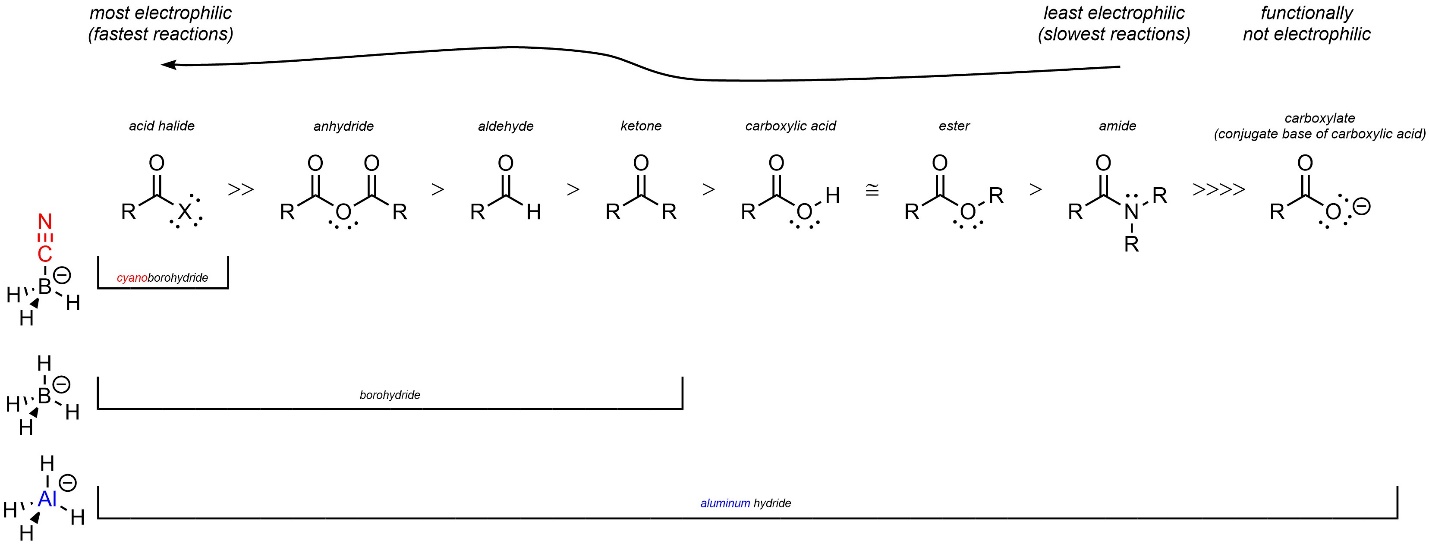
Figure 13.7 – Comparison of Carbonyl-Containing Functional Groups Able to be Reduced by Cyanoborohydride, Borohydride, and Aluminum Hydride.
These differences can be exploited when a molecule has multiple carbonyl-containing functional groups (see Section 13.8.5).
13.8.2. Reaction: Reduction of Carboxylic Acids
It is possible to convert a carboxylic acid into a primary alcohol using a very nucleophilic hydride source, typically lithium aluminum hydride (LiAlH4; Scheme 13.33). Because this is done using aluminum hydride it requires a polar aprotic solvent, typically tetrahydrofuran (THF), and must be quenched using a strong acid or base to generate the final product.
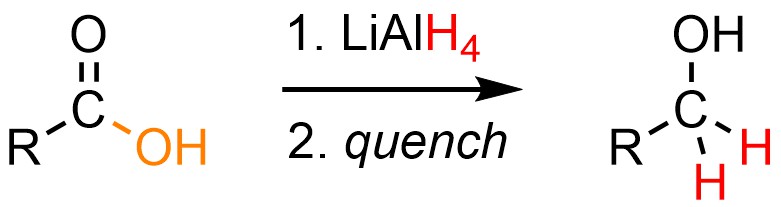
Scheme 13.33 – Generalized Reaction Equation for Converting Carboxylic Acids to Primary Alcohols Using Lithium Aluminum Hydride (LAH).
Recall that lithium aluminum hydride may deliver up to four equivalents of hydride. During this process it may acquire different substituents (see Section 7.3.2), which can make depictions of the reaction mechanism complex. For convenience the following mechanism will show a different molecule of aluminum hydride delivering each hydride, but this is not likely to be accurate. The mechanism for this reaction is straightforward but time consuming to draw. Many sources do not keep track of all compounds in the reaction, adding or removing them as needed. At an introductory level this is acceptable but discouraged.
First, aluminum hydride (base) removes a proton from the carboxylic acid (acid; Scheme 13.34). The anionic oxygen (nucleophile) attacks the aluminum (electrophile). This significantly increases its leaving group ability. Depending on the exact reaction occurring it is likely that these two steps actually happen at the same time through a slightly different process. At an introductory level it is acceptable to draw them as separate steps. Another hydride (nucleophile) attacks the carbonyl (electrophile). This forms a new bond and adds a lone pair to the oxygen. Then the lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group. A third hydride (nucleophile) attacks the new carbonyl (electrophile). Finally, the anionic oxygen (nucleophile) attacks the aluminum (electrophile). After the reaction is complete a strong acid or base is added to quench, forming the final product.
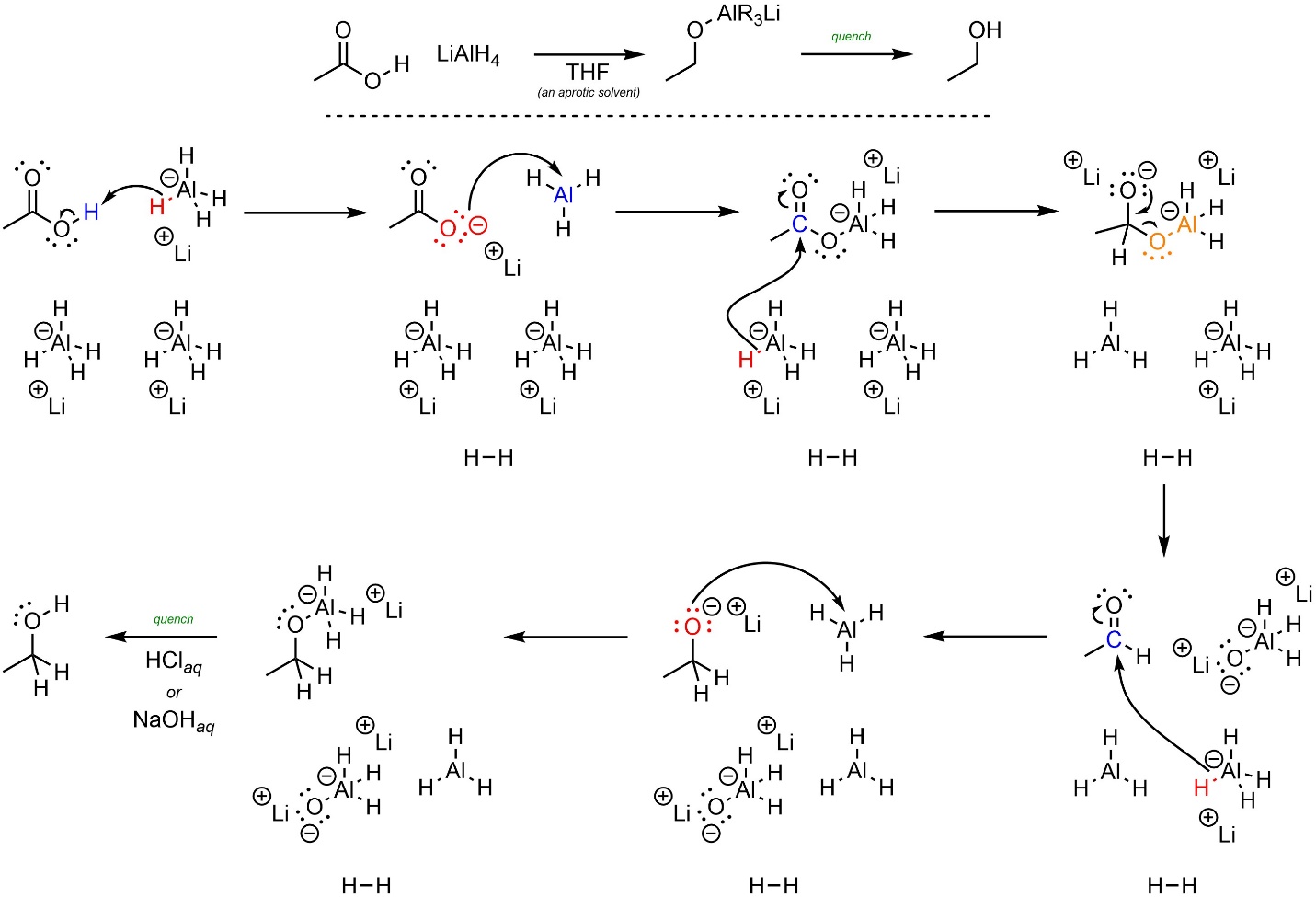
Scheme 13.34 – Reaction Mechanism for Addition-Elimination Followed by Addition of Ethanoic Acid with Lithium Aluminum Hydride.
Converting a carboxylic acid to an alcohol requires three equivalents of hydride.
13.8.3. Reaction: Reduction of Acid Halides, Anhydrides, and Esters
It is possible to convert an acid halide, anhydride, or ester into a primary alcohol using a moderate or strong nucleophilic hydride source, typically sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4; Scheme 13.35). The solvent used must match the hydride source used (see Section 7.3.2), though often tetrahydrofuran (THF) is used in either case. The reaction must be quenched using a strong acid or base to generate the final product.
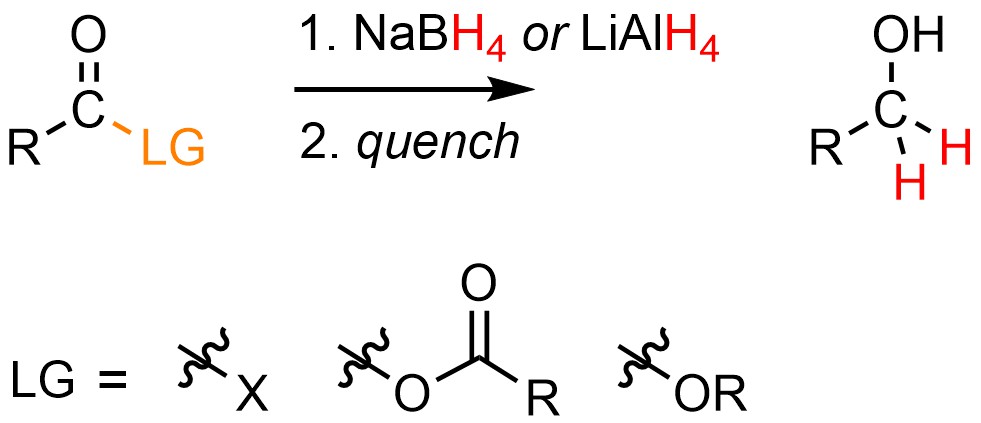
Scheme 13.35 – Generalized Reaction Equation for Converting Acid Halides, Anhydrides, or Esters to Primary Alcohols Using Sodium Borohydride or Lithium Aluminum Hydride (LAH).
For convenience the following mechanism will show a different molecule of borohydride delivering each hydride, but this is not likely to be accurate. The mechanism for this reaction is straightforward but time consuming to draw. Many sources do not keep track of all compounds in the reaction, adding or removing them as needed. At an introductory level this is acceptable but discouraged.
The mechanism for these reactions follows the standard addition-elimination mechanism followed by a standard addition mechanism (Scheme 13.36). First, a hydride (nucleophile) attacks the carbonyl (electrophile). This forms a new bond and adds a lone pair to the oxygen. Then the lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group. A second hydride (nucleophile) attacks the new carbonyl (electrophile). Finally, the anionic oxygen (nucleophile) attacks the boron or aluminum (electrophile). After the reaction is complete a strong acid or base is added to quench, forming the final product.
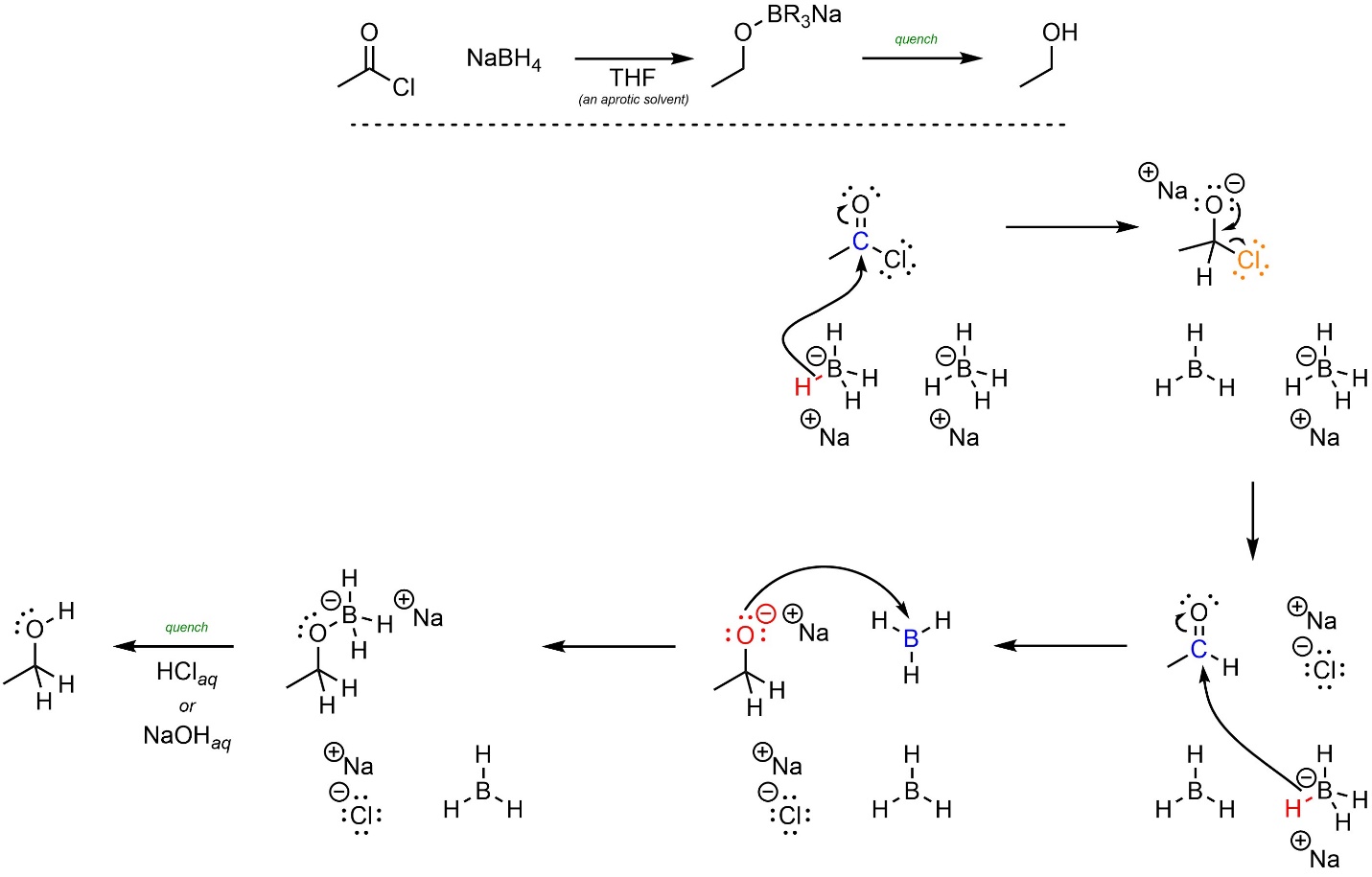
Scheme 13.36 – Reaction Mechanism for Addition-Elimination Followed by Addition of Ethanoyl Chloride with Sodium Borohydride.
Converting an acid halide, anhydride, or ester to an alcohol requires two equivalents of hydride.
Recall that sodium cyanoborohydride (NaBH3CN) is only nucleophilic enough to reduce acid halides. It is possible to convert an acid halide into an aldehyde using a weak nucleophilic hydride source, typically sodium cyanoborohydride (NaBH3CN; Scheme 13.36). This follows the same mechanism (see Scheme 13.37) but ends after the first two steps; sodium cyanoborohydride is not nucleophilic enough to attack the carbonyl of an aldehyde.
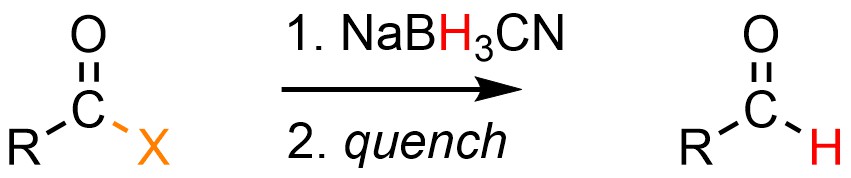
Scheme 13.37 – Generalized Reaction Equation for Converting Acid Halides to Aldehydes Using Sodium Cyanoborohydride.
Converting an acid halide to an aldehyde requires one equivalent of hydride.
13.8.4. Reaction: Reduction of Amides
The reduction of amides follows a slightly unusual path: it is possible to convert an amide into an amine using a very nucleophilic hydride source, typically lithium aluminum hydride (LiAlH4; Scheme 13.38). Because this is done using aluminum hydride it requires a polar aprotic solvent, typically tetrahydrofuran (THF). Unlike most aluminum hydride reductions these do not require a quench to generate the final product.
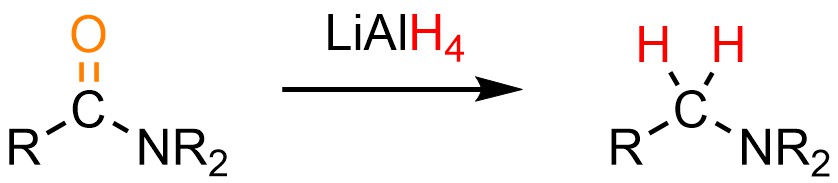
Scheme 13.38 – Generalized Reaction Equation for Converting Amides to Amines Using Lithium Aluminum Hydride (LAH).
Recall that nitrogen atoms have unusual properties, including that they are much stronger bases than they are nucleophiles. A consequence of this is that anionic nitrogen atoms are extremely poor leaving groups (because they are very strong bases). This affects the reactivity of amides with hydride sources.
For convenience the following mechanism will show a different molecule of aluminum hydride delivering each hydride, but this is not likely to be accurate. The mechanism for this reaction is straightforward but time consuming to draw. Many sources do not keep track of all compounds in the reaction, adding or removing them as needed. At an introductory level this is acceptable but discouraged.
First, aluminum hydride (nucleophile) attacks the carbonyl (electrophile; Scheme 13.39). This forms a new bond and adds a lone pair to the oxygen. Instead of returning and ejecting an anionic nitrogen leaving group, the anionic oxygen (nucleophile) attacks the aluminum (electrophile). This significantly increases its leaving group ability. Then the lone pair of the nitrogen forms a π bond with the carbon, ejecting the leaving group. This forms an iminium, which has similar structure and reactivity to an activated carbonyl. Finally, another hydride (nucleophile) attacks the iminium (electrophile), generating the final product.
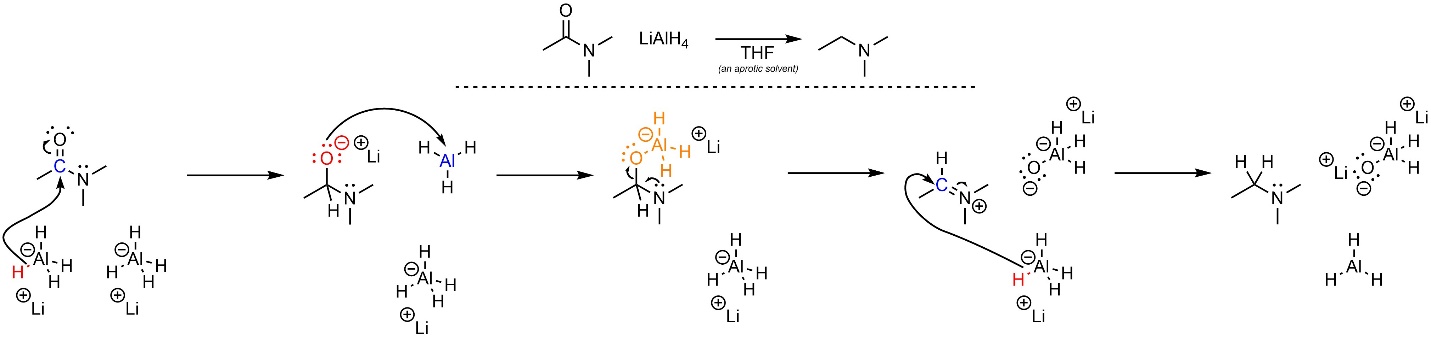
Scheme 13.39 – Reaction Mechanism for Addition-Elimination Followed by Addition of N,N-Dimethylethanamide with Lithium Aluminum Hydride.
Converting an amide to an amine requires two equivalents of hydride.
13.8.5. Practical Applications of Using Different Hydride Sources
The primary benefit of having multiple hydride sources with differing nucleophilicities is that a single starting material can be used to generate multiple products depending on which hydride source is used (Scheme 13.40). This has few consequences at an introductory level beyond recognizing which groups can react with which hydride sources and what they form (this also makes it very useful for examination purposes). However, this is particularly valuable for the synthesis of complex compounds and is frequently taken advantage of in syntheses of pharmaceuticals and other natural products.
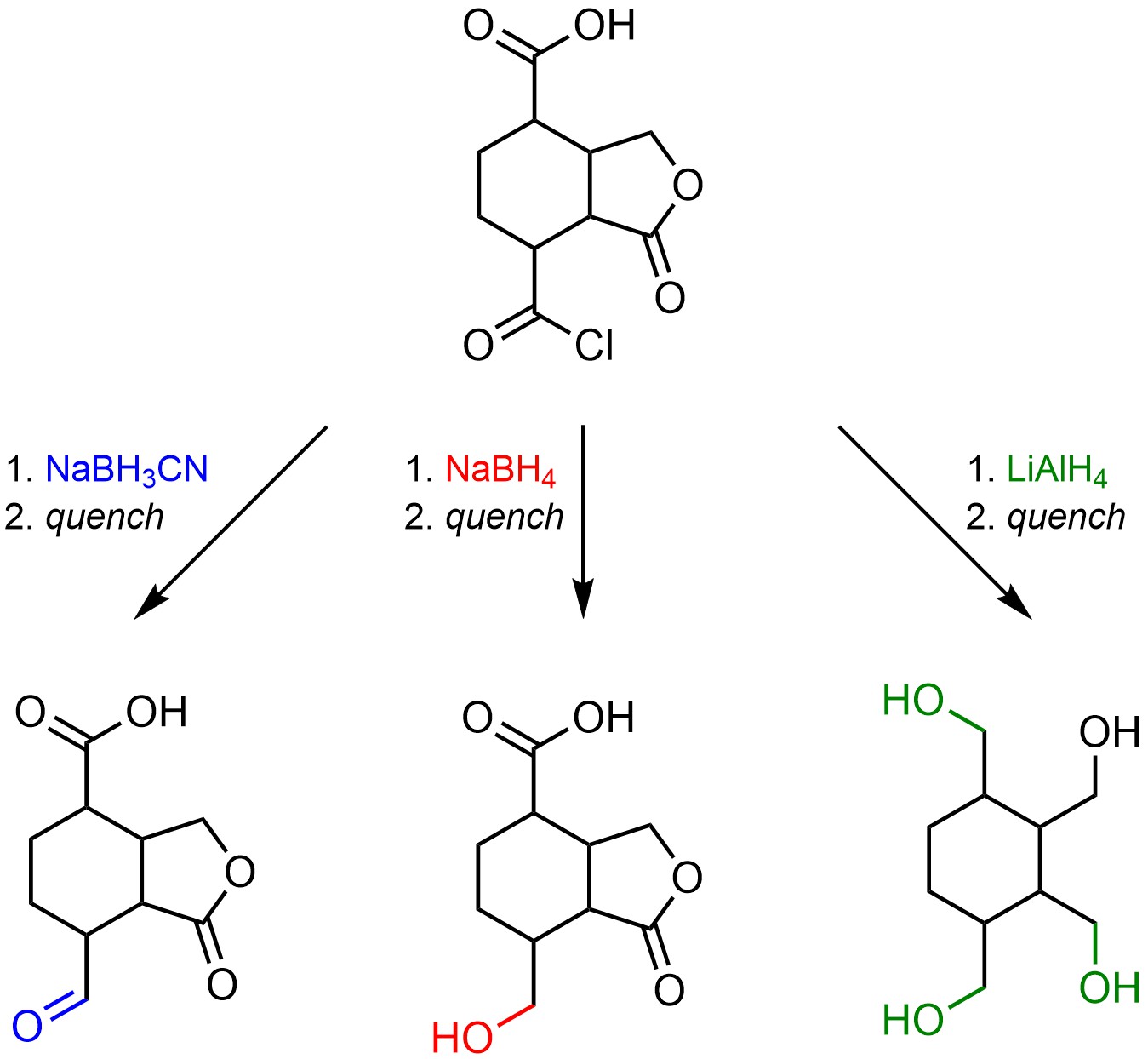
Scheme 13.40 – Example Highlighting Different Reaction Outcomes for Reduction of a Compound with Multiple Carbonyl-Containing Functional Groups Using Different Hydride Sources.

