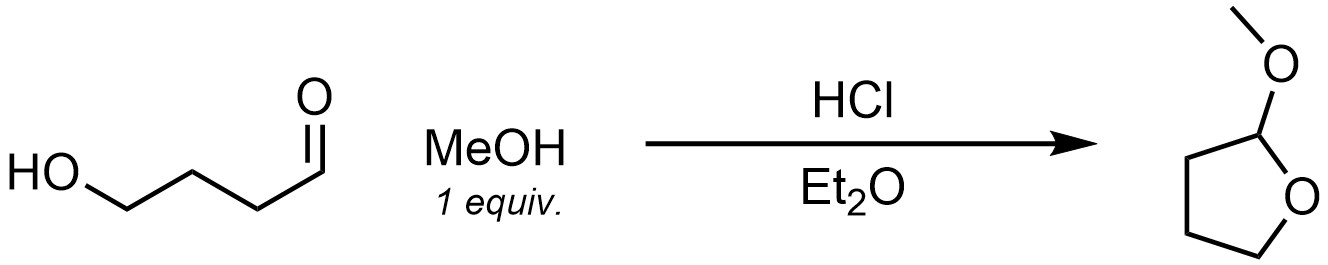
Chapter 7 Practice Problems
Answers for these practice problems are on the next page.
A good approach is to answer all of the questions on a piece of paper and then check your answers. This avoids accidentally seeing the answer(s) for questions you have not done yet.
Q7.1: In each of the following reactions identify (circle and label, draw arrows to, etc.) the nucleophile and electrophile for the reaction. Indicate in some way which atoms specifically are the relevant nucleophilic and electrophilic ones for the reaction taking place.
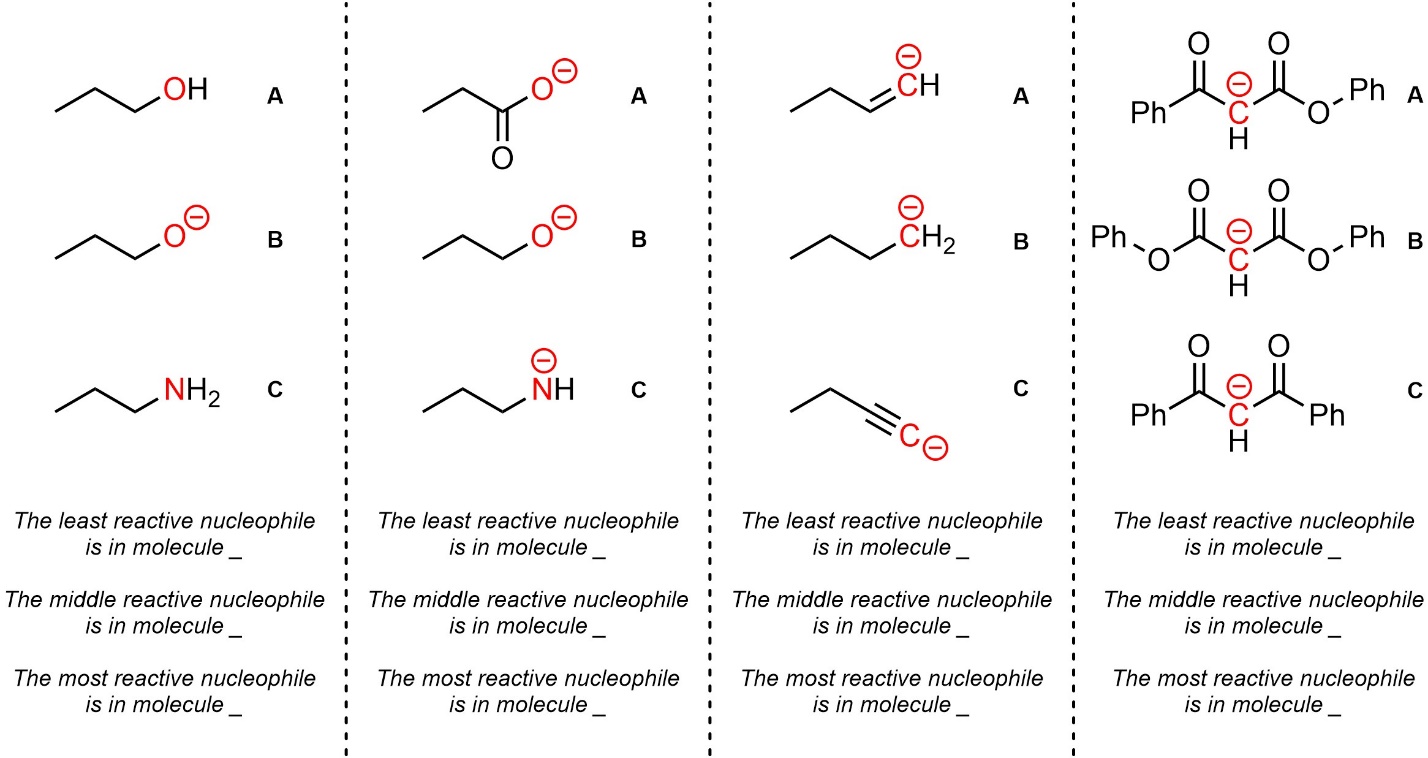
Q7.2: Rank each set of nucleophiles in increasing order of reactivity (least to most). The nucleophilic atoms to consider are highlighted.
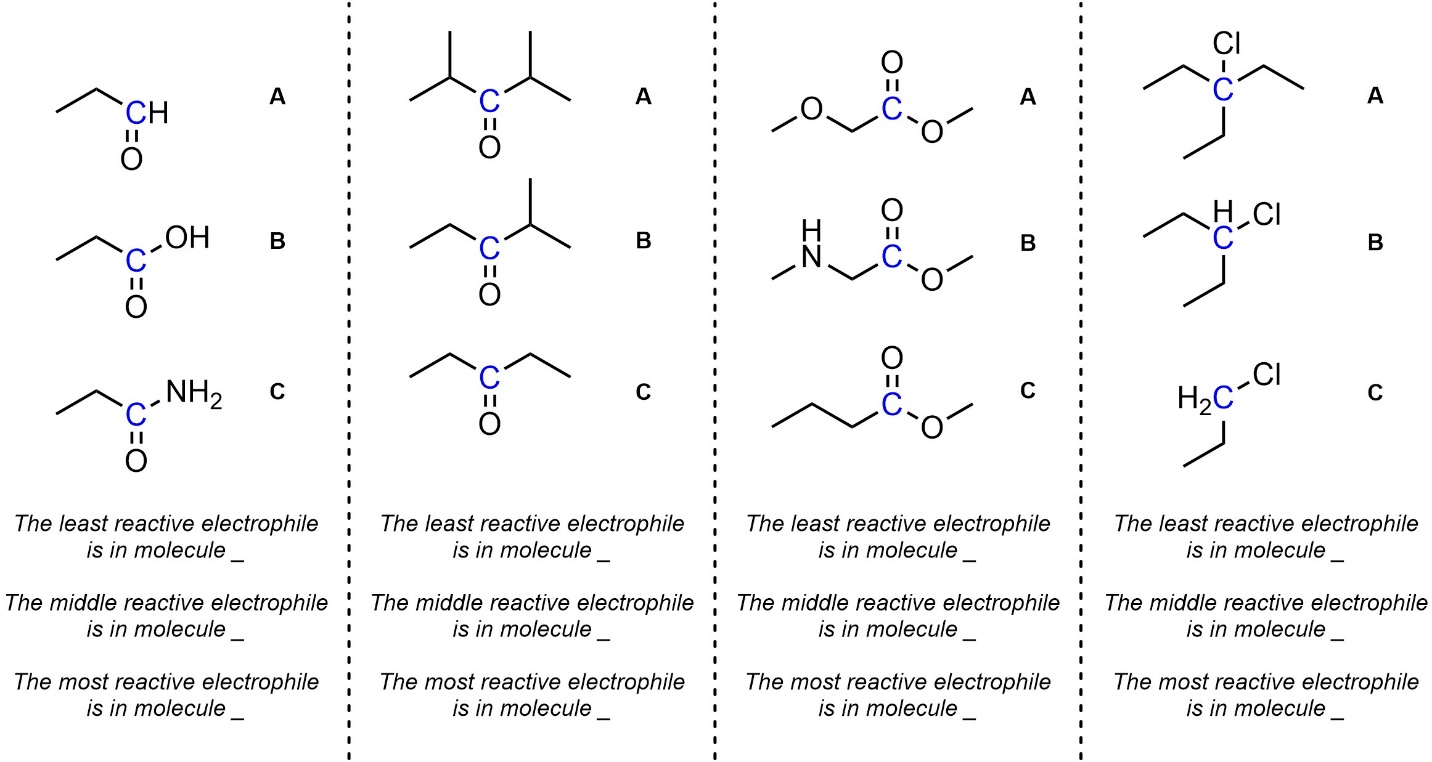
Q7.3: Rank each set of electrophiles in increasing order of reactivity (least to most). The electrophilic atoms to consider are highlighted.
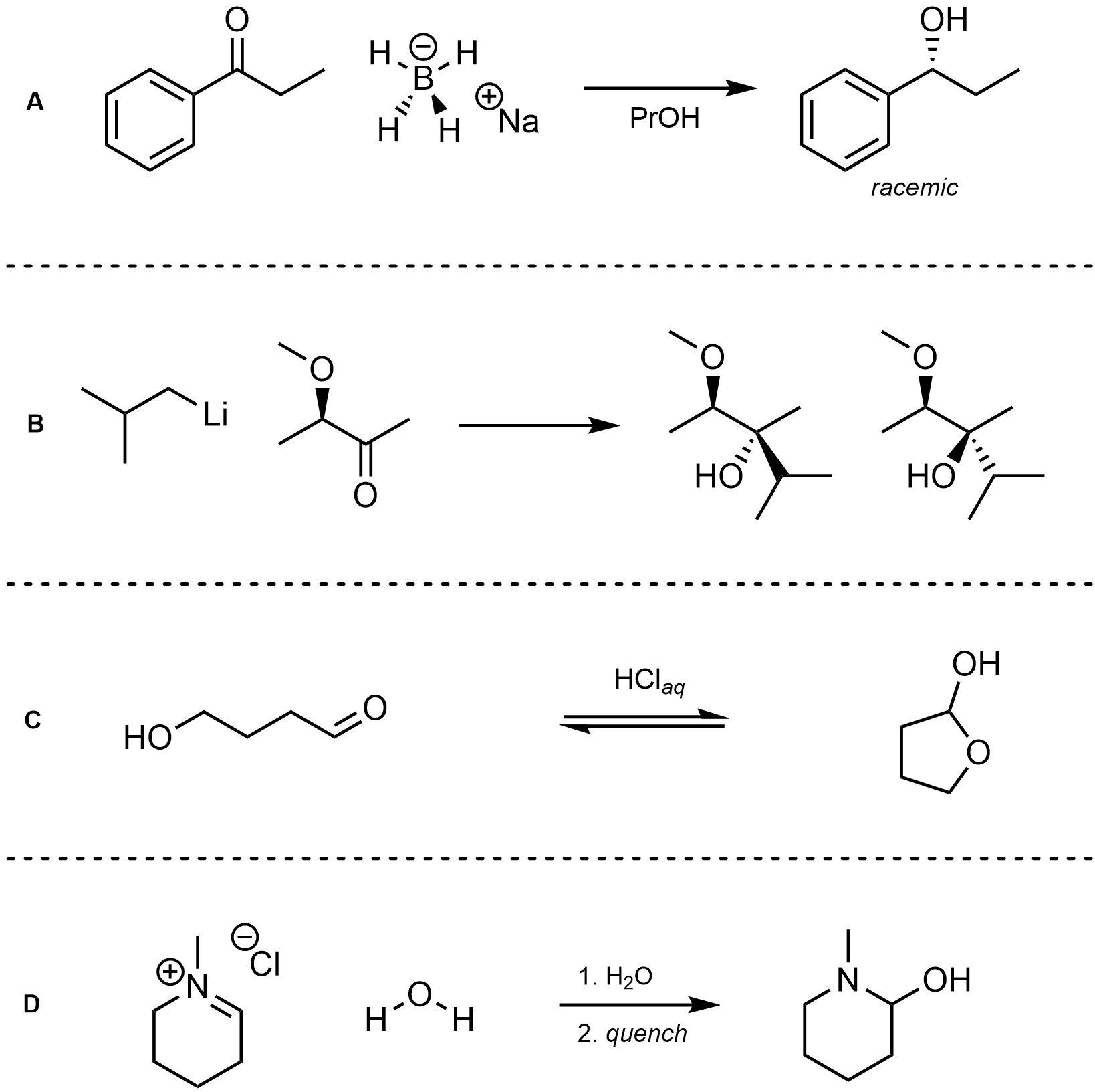
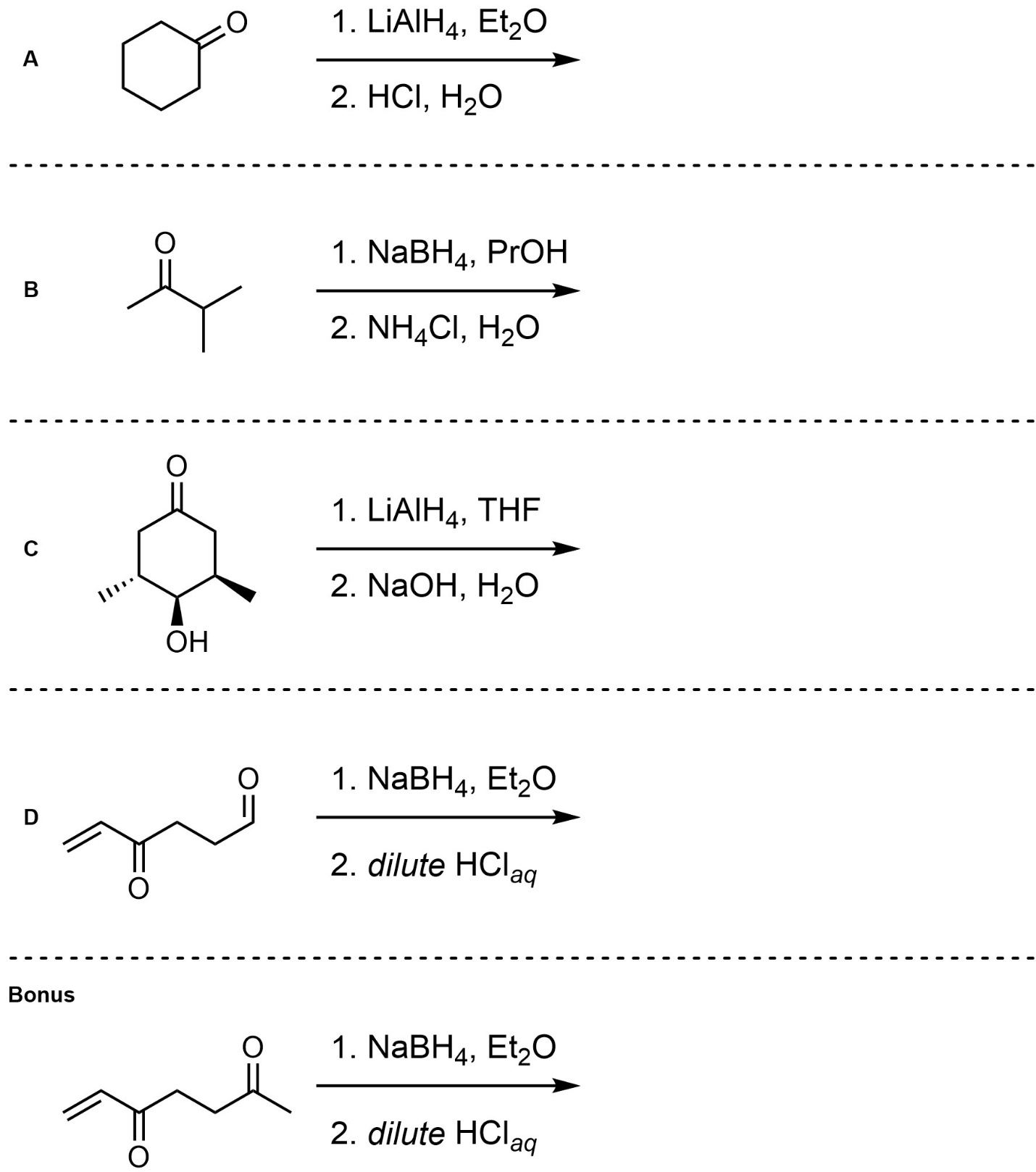
Q7.4: For each reaction draw the expected major organic product(s). The products must be drawn as line-angle structures. Clearly indicate the appropriate stereochemistry using hashed and wedged bonds where necessary. If the product(s) is/are formed as more than one diastereomer, draw all diastereomers formed. If the product(s) is/are formed as a racemate (racemic mixture) draw only one enantiomer and write the word “racemic” beneath/beside it. Do not write “racemic” if it does not apply.
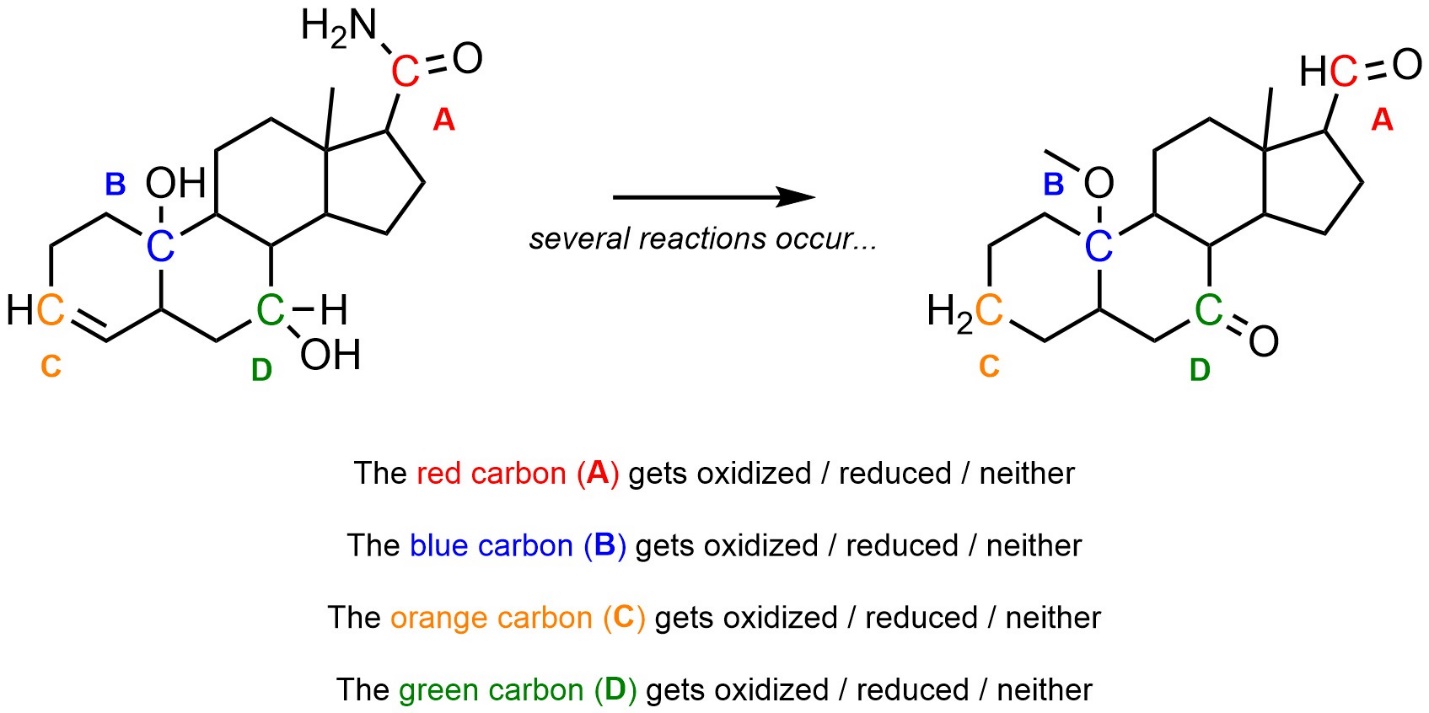
Q7.5: Class each transformation as reduction or oxidation.
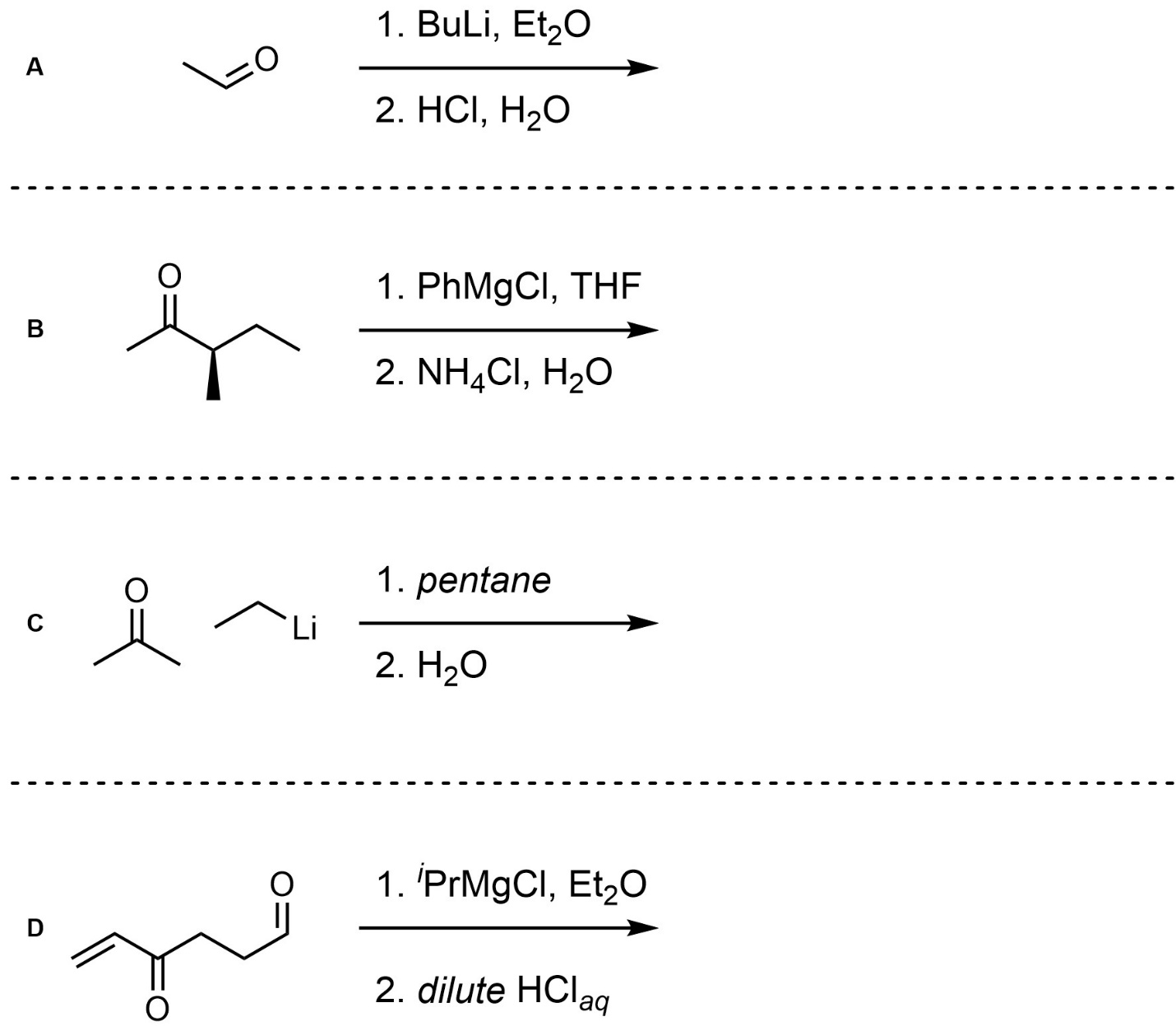
Q7.6: For each reaction draw the expected major organic product(s). The products must be drawn as line-angle structures. Clearly indicate the appropriate stereochemistry using hashed and wedged bonds where necessary. If the product(s) is/are formed as more than one diastereomer, draw all diastereomers formed. If the product(s) is/are formed as a racemate (racemic mixture) draw only one enantiomer and write the word “racemic” beneath/beside it. Do not write “racemic” if it does not apply.
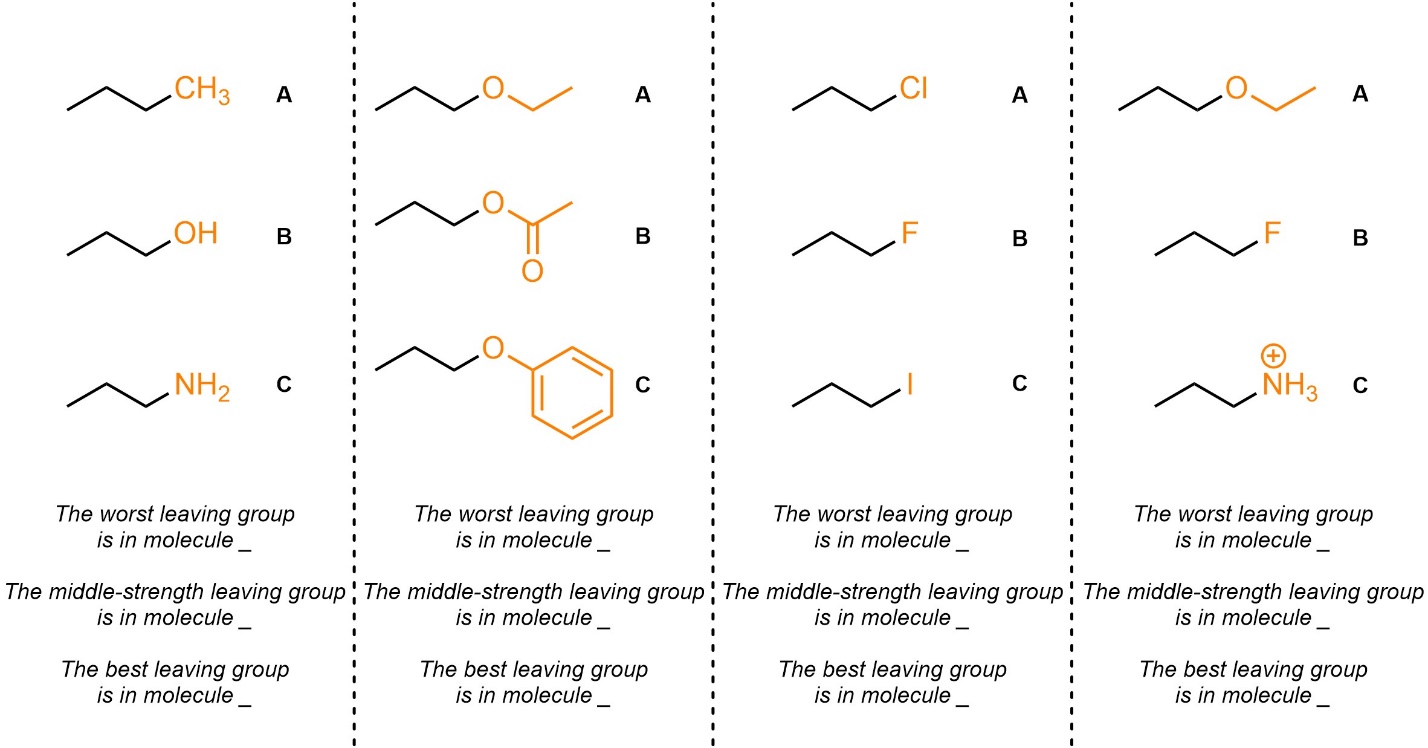
Q7.7: Rank each set of leaving groups in increasing order of leaving group ability (worst to best). The leaving groups to consider are highlighted.
Q7.8: A reaction equation is given below. Write a brief sentence explaining which compound is the catalyst and how the catalyst will increase the reaction rate. Then propose a reasonable mechanism for the reaction. Show all necessary intermediates, curved arrows, lone pairs, and formal charges. Remember to regenerate the catalyst at the end of the reaction.
Note: For practice purposes this reaction is intentionally chosen to be challenging and have a semi-lengthy mechanism to write out. Hint: Think critically about what functional group is being made in the product.