13.2. Concept and General Form
The primary difference between functional groups that contain a carbonyl directly attached to a heteroatom and aldehydes/ketones lies in what can happen after a nucleophilic attack on the carbonyl. Recall that carbanions and hydrides are very powerful bases. As a result, they are very poor leaving groups. When a nucleophile attacks an aldehyde or ketone it undergoes an addition reaction and stops (Scheme 13.1). However, if the hydrogen/carbon is replaced with a heteroatom then instead of being a very poor leaving group it can range from being poor to excellent. The intermediate can collapse back down to a new carbonyl-containing functional group and eject the leaving group.

Scheme 13.1 – Comparison of Reaction Mechanisms for Sodium Methoxide Attacking Acetone and Sodium Methoxide Attacking Ethanoyl Chloride.
These reactions are sometimes referred to as addition-elimination reactions (despite the second step not being a true elimination). Some texts may refer to them as acylation or acyl substitution reactions.
The typical outcome of an addition-elimination (acylation) reaction is to convert one carbonyl-containing functional group into another (Scheme 13.2).
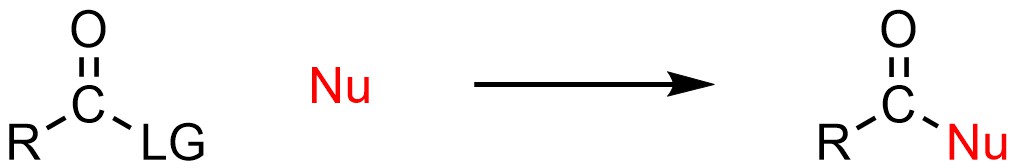
Scheme 13.2 – Generalized Reaction Equation for Addition-Elimination Reactions (Acylations).
Not all interconversions are possible. Of those that are, some require catalysis or special reagents, and some follow unusual and lengthy mechanisms. There are dozens of reactions following this general principle, and many specifically named addition-elimination reactions. This text will focus on the most common types of addition-elimination reactions and generally avoid using the specific names. It is possible that they may be encountered in assignments, labs, discussions, etc. In these cases, searching the name of the reaction using a resource such as Wikipedia will be a straightforward way of understanding what is being discussed.

