13.6. Reactions with Anhydride Electrophiles
Anhydrides are a relatively uncommon functional group in nature but are semi-commonly used in laboratory syntheses. They are slightly less electrophilic than acid halides. As a result, they are capable of undergoing many similar reactions but have fewer potential side reactions (a discussion of possible side reactions in addition-elimination reactions lies outside the scope of this text).
Anhydrides are generally subdivided into two classes: symmetric and asymmetric (sometimes called mixed anhydrides; Figure 13.5). Mixed anhydrides have very interesting chemical reactivities and may undergo some reactions that symmetric anhydrides cannot. However, their altered chemical reactivity makes them significantly more complex to discuss. At an introductory level only an understanding of symmetric anhydrides is required; this text will not feature examples of mixed anhydrides.
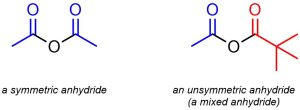
Figure 13.5 – Examples of a Symmetric Anhydride, Ethanoic anhydride, and an Unsymmetric (Mixed) Anhydride, Ethanoic pivalic anhydride.
13.6.1. Reaction: Converting Anhydrides to Carboxylic Acids/Esters
It is possible to convert an anhydride into a carboxylic acid or ester. This can be done using neutral nucleophiles like water (H-OH) or alcohols (H-OR) but it is more common to use anionic ones such as sodium hydroxide (NaOH) or a sodium alkoxide (NaOR; Scheme 13.12).
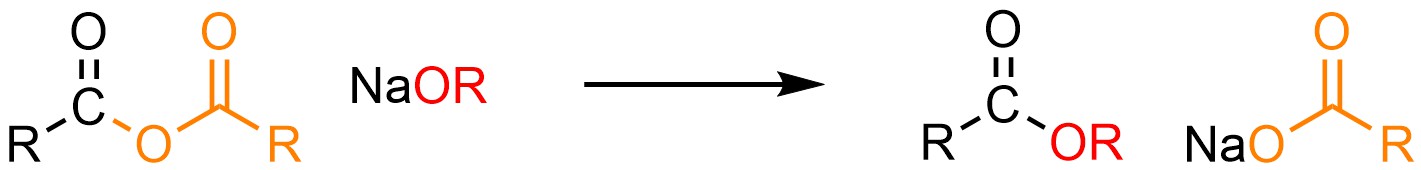
Scheme 13.12 – Generalized Reaction Equation for Addition-Elimination Converting Anhydrides to Carboxylic Acids or Esters.
When discussing the “product” of these reactions only the carbonyl-containing group that acted as the electrophile is normally considered. For example, if the reaction is quenched the leaving group component will become a carboxylic acid in both cases. This does not normally have any consequences for the reaction and is typically ignored.
13.6.1.1. Mechanism
This reaction follows the standard two step mechanism for addition-elimination reactions (see Section 13.3; Scheme 13.13). The nucleophile (alkoxide) attacks the electrophile (anhydride). This forms a new bond and adds a lone pair to the oxygen. Then that lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group (carboxylate).
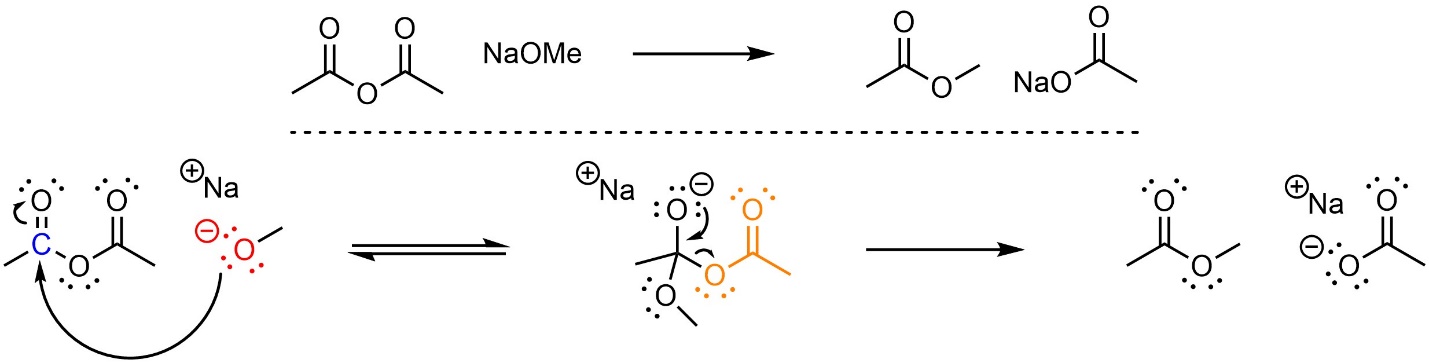
Scheme 13.13 – Reaction Mechanism for Addition-Elimination (Acylation) of Sodium Methoxide with Ethanoic Anhydride.
13.6.2. Reaction: Converting Anhydrides to Amides
It is possible to convert an anhydride into an amide. Anionic nitrogen atoms have unusual reactivity: they are much more basic than they are nucleophilic. Why this is true is beyond the scope of this text. As a result of their unusual reactivity, this can only be done using neutral nucleophiles like amines (H-NR2; Scheme 13.14).
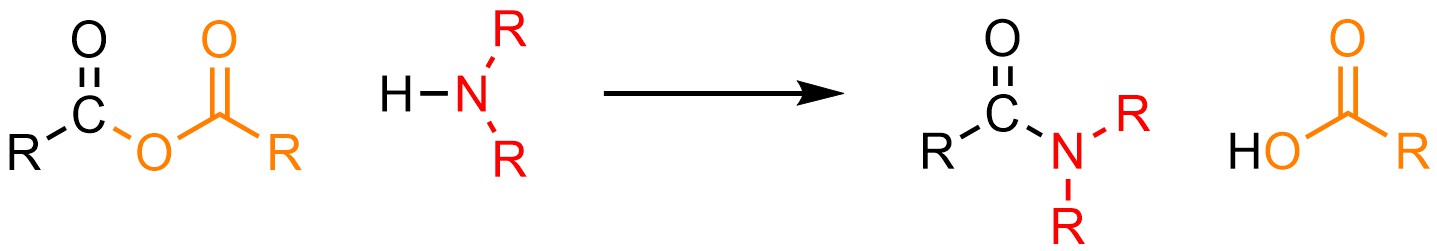
Scheme 13.14 – Generalized Reaction Equation for Addition-Elimination Converting Anhydrides to Amides.
As with the synthesis of amides from acid halides (see Section 13.5.3) the side product of this reaction is an acid (carboxylic acid; HO(CO)R) instead of a salt (NaO(CO)R). As a result, the same special considerations must be made; the reaction must be carried out using two equivalents of the amine or an equivalent of a non-nucleophilic base must be added.
13.6.2.1. Mechanism
This reaction follows the standard two step mechanism for addition-elimination reactions (see Section 13.3) followed by a deprotonation (Scheme 13.15). The nucleophile (amine) attacks the electrophile (anhydride). This forms a new bond and adds a lone pair to the oxygen. Then that lone pair comes back and reforms the π bond with the carbon, ejecting the leaving group (carboxylate). Finally, a base (amine) removes a proton to generate the final product and a salt.
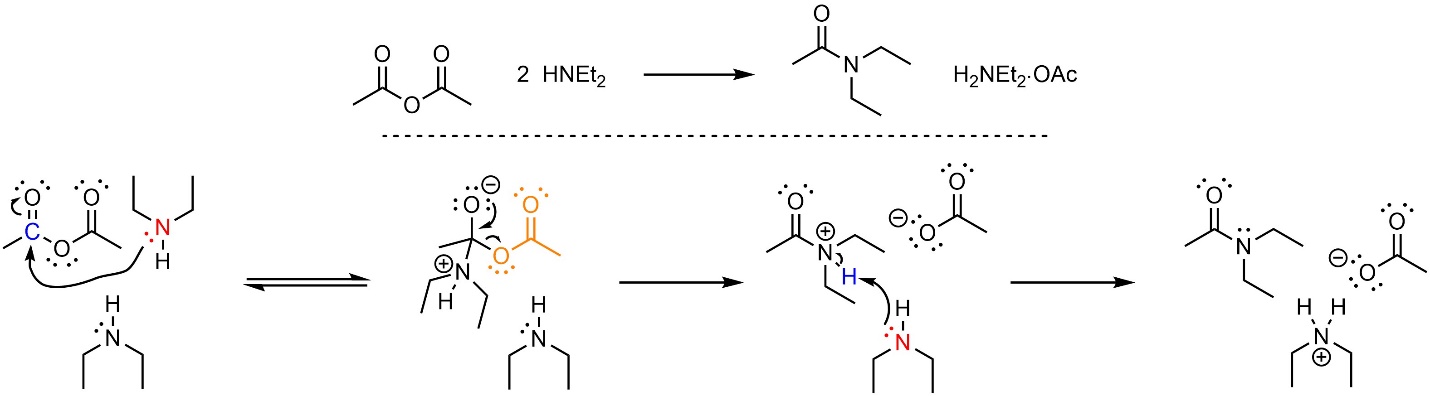
Scheme 13.15 – Reaction Mechanism for Addition-Elimination (Acylation) of Diethylamine with Ethanoic Anhydride Using Two Equivalents of Amine.

