4.5. Adding Stereochemical Information to IUPAC Names
Absolute configurations are required in an IUPAC name to ensure that the correct stereoisomer is conveyed. First, name the molecule using the standard rules (see Section 2.5). Then, all stereochemical information is prepended to the prefix in a single bracketed term. Use the numbering of the skeleton to specify which stereocentre and/or alkene is being described, followed immediately by the configuration. Technically, if there is only one configuration of each type the numerical descriptor is not needed. Adding these numerical descriptors to the name is always acceptable, but being aware of this rule may help when encountering examples that omit them. If there are multiple configurations (multiple stereocentres and/or alkenes) separate each with a comma. Configurations are listed in numerical order (Figure 4.37).
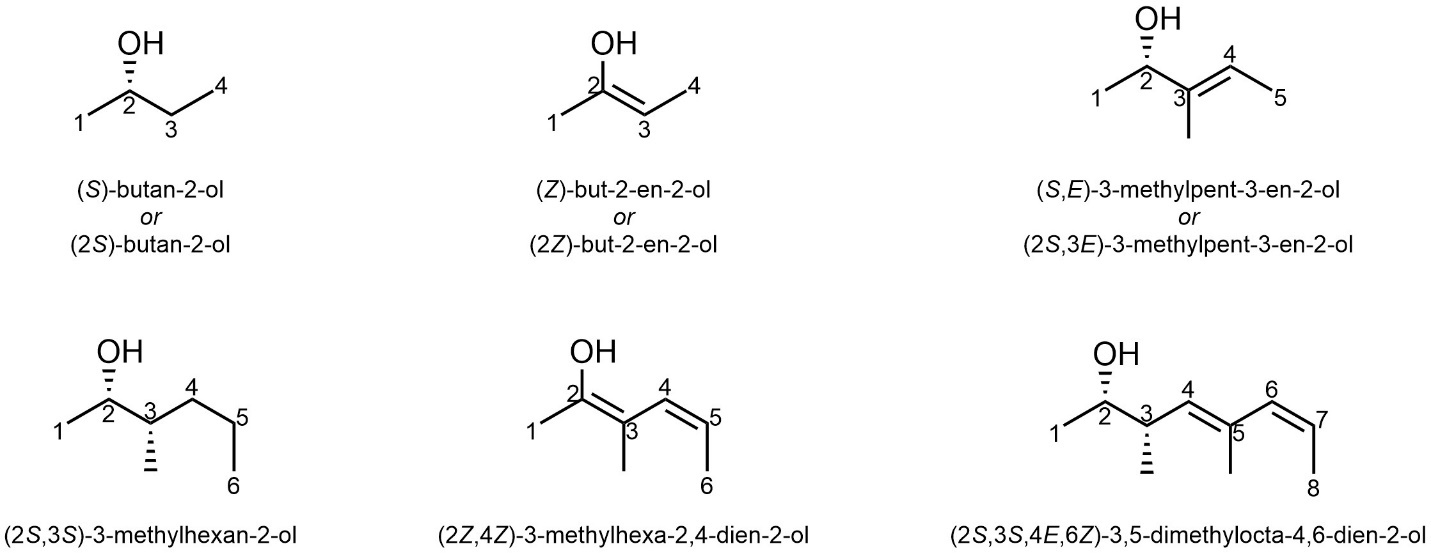
Figure 4.37 – Examples of Compounds Named Using the IUPAC system with Stereochemical Information.

