8.10. Reaction: Addition of HO-OH or HO-OR
It is possible to add an alcohol (OH) and an alcohol or ether (OH or OR) across an alkene (Scheme 8.32). This is accomplished by first forming the epoxide and then opening the ring by attacking it with either water or an alcohol in the presence of an acid or base catalyst.
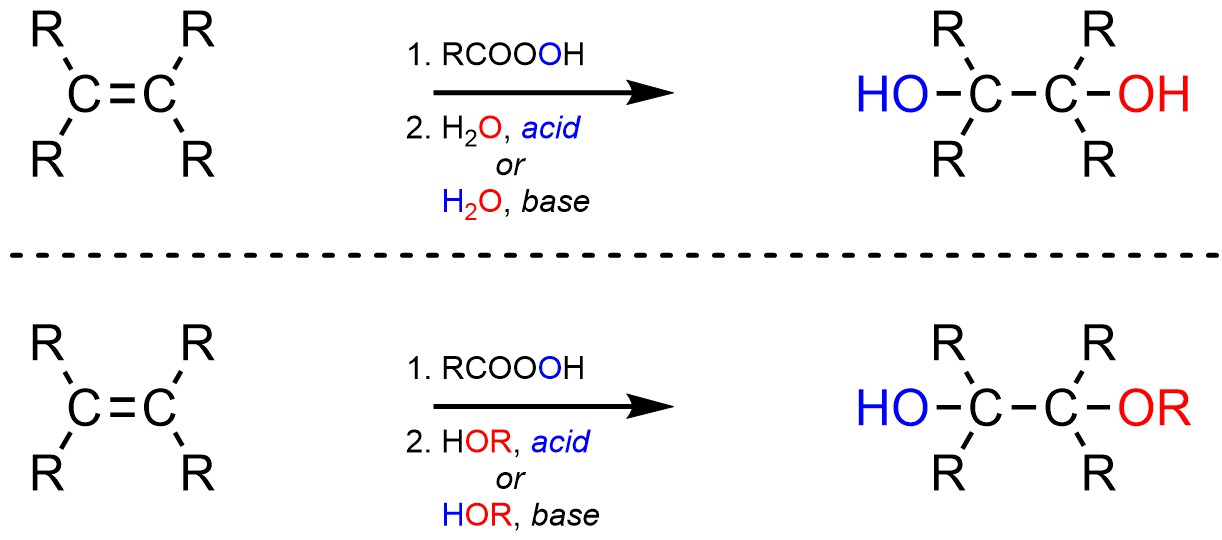
Scheme 8.32 – Generalized Reaction Equations for Addition of X-OH or X-OR Across an Alkene.
8.10.1. Acid-Catalyzed: Mechanism and Regioselectivity
In the first reaction the epoxide is generated using a peroxyacid (see Schemes 8.30 and 8.31). Then, acid-catalyzed ring-opening follows a similar pattern to other acid-catalyzed nucleophile-electrophile reactions (Scheme 8.33). The strong acid reacts with water or an alcohol to form a cationic oxygen (an oxonium, step not shown). This is the active catalyst. First, the electrophile (epoxide) gets activated by the catalyst. This greatly increases its electrophilicity. Second, the water or alcohol (nucleophile) attacks the activated electrophile. This creates a new C-O bond, an alcohol, and a new oxonium intermediate. Finally, another equivalent of water or alcohol removes a proton, generating the final product and regenerating the catalyst.
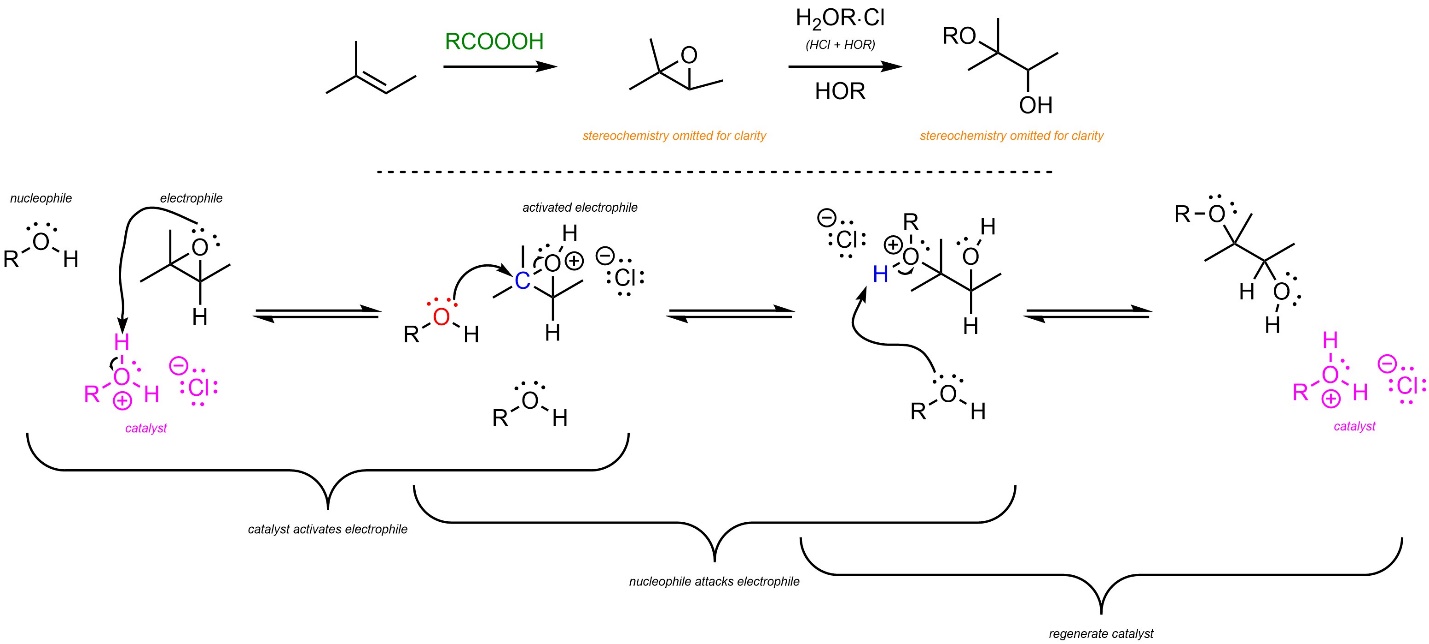
Scheme 8.33 – Reaction Mechanism for Acid-Catalyzed Ring Opening of the Epoxide Formed from 2-Methylbut-2-ene.
As before, the major regioisomer will be the Markovnikov product. This is due to the development of a partial cationic charge on the carbon atom during the transition state, which has a similar effect to an energy difference in carbocation intermediates.
8.10.2. Base-Catalyzed: Mechanism and Regioselectivity
In the first reaction the epoxide is generated using a peroxyacid (see Schemes 8.30 and 8.31). Then, base-catalyzed ring-opening follows a similar pattern to other base-catalyzed nucleophile-electrophile reactions (Scheme 8.34). The strong base reacts with water or an alcohol to form an anionic oxygen (a hydroxide or alkoxide, step not shown). This is the active catalyst. First, the nucleophile (water or alcohol) gets activated by the catalyst. This greatly increases its nucleophilicity. Second, the activated nucleophile attacks the epoxide (electrophile). This creates a new C-O bond and an alkoxide intermediate. Finally, another equivalent of water or alcohol is deprotonated, generating the final product and regenerating the catalyst.
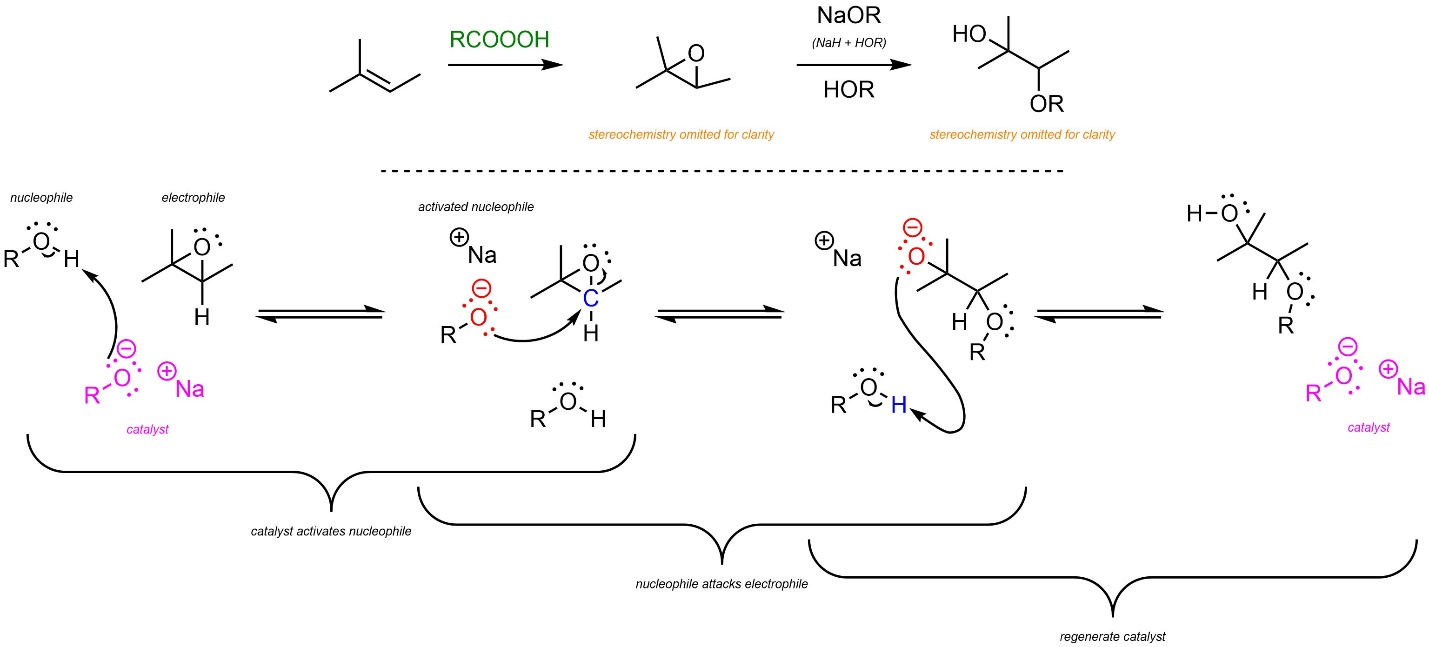
Scheme 8.34 – Reaction Mechanism for Base-Catalyzed Ring Opening of the Epoxide Formed from 2-Methylbut-2-ene.
Unlike before, the major regioisomer will be the Anti-Markovnikov product. The nucleophile is now so nucleophilic that steric strain from the alkyl groups of the more substituted position has more of an effect than the relative stability of the carbocations during the transition states. Regioselectivity can thus be controlled by using either acid or base catalysis.
8.10.3. Stereoselectivity – Stereospecific: trans
Additions of HO-OR across alkenes are stereospecific (diastereospecific). All stereoselectivity considerations for this reaction are identical to those for the addition of X-X across an alkene (see Section 8.7.3.). It is only possible for the water/alcohol to add to the carbon by attacking from the opposite side of the epoxide (trans addition; Scheme 8.35). This is true for both acid-catalyzed and base-catalyzed ring opening reactions.
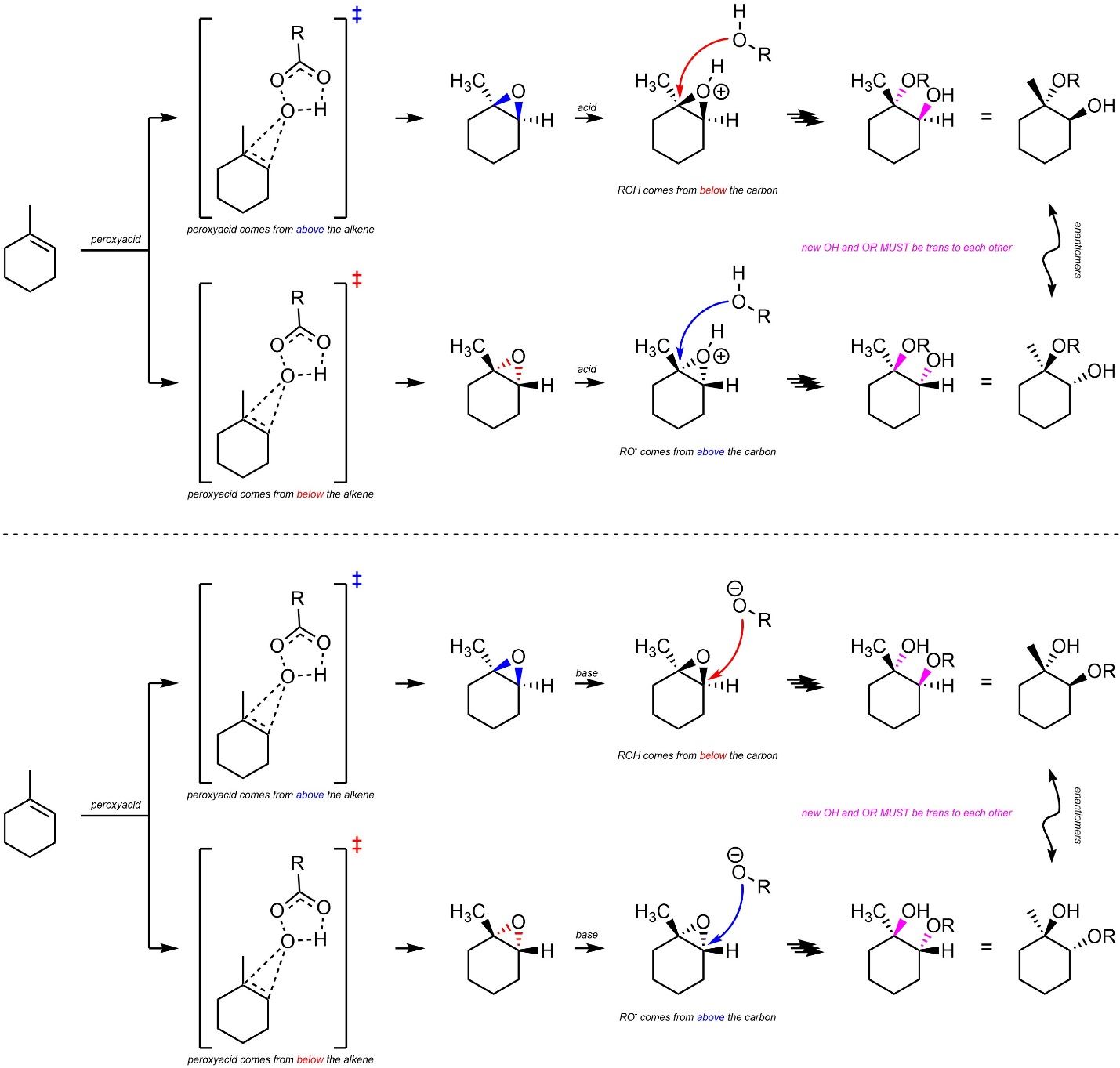
Scheme 8.35 – Diastereospecific Addition of HO-OR Across the Alkene of Methylcyclohexene as Catalyzed by Acid or Base.
It is very important to remember that this is a form of diastereoselectivity (by being diastereospecific). There is no enantioselectivity.

