2.2. Functional Groups
To simplify their work chemists look for patterns in molecules. A functional group is a collection of specific atoms connected in a specific way. Since structure directly dictates reactivity, functional groups behave VERY similarly (often identically) in each molecule in which they are found.
It is sometimes convenient to think of functional groups as building blocks, like Lego pieces. Each type of brick has a specific shape. The individual blocks can be connected together in many ways and have other blocks around them. However, a red 2×2 brick will still be a red 2×2 brick if it is in a model of a house or a model of a car. Where a brick is placed might (or might not) affect how it can interact with other pieces. Some pieces can only be attached at certain points, etc. The goal, for now, is to be able to identify the specific building blocks (functional groups) and name them.
There are infinitely many molecules that can be constructed with any given functional group. For example, all of these molecules contain an aldehyde (Figure 2.2).
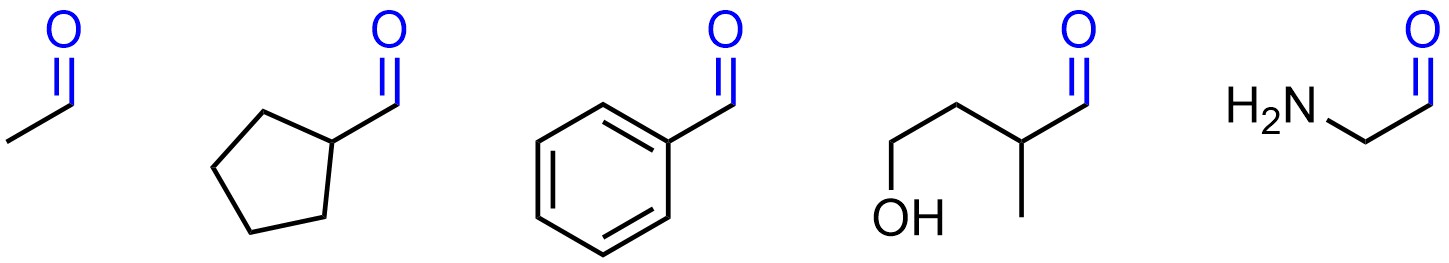
Figure 2.2 – Examples of Molecules Containing Aldehyde Functional Groups.
Since we are often only talking about the functional groups, we find it convenient to use the letter ‘R’ to abbreviate part of a molecule. This allows us to be generic (and faster) in our discussion since the extra parts of the molecule are not relevant (Figure 2.3).
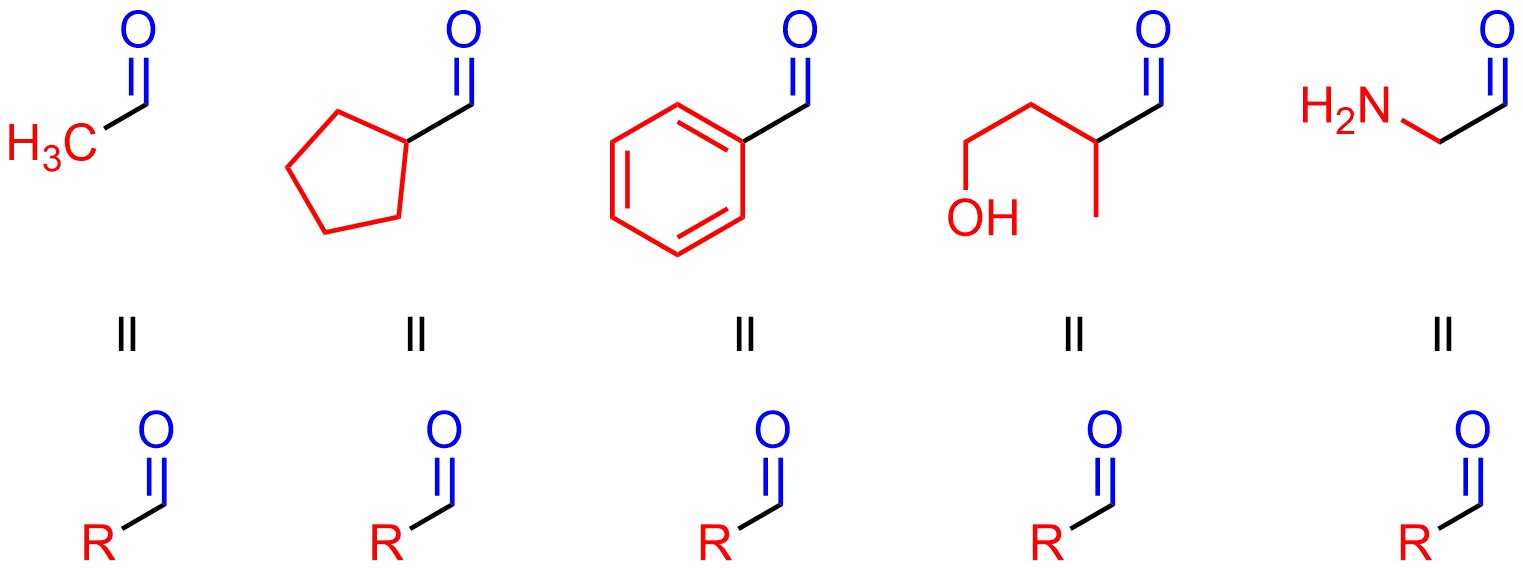
Figure 2.3 – Abbreviation of Molecules Containing Aldehyde Functional Groups Using ‘R’.
Confusingly, the letter ‘R’ is sometimes used to mean “any set of atoms is possible here” and sometimes used to mean “a specific set of atoms is possible here”. For example, if R = O or N (with other atoms attached to the oxygen/nitrogen), then this is NOT an aldehyde functional group anymore (Figure 2.4). In all sections of 2.2 assume ‘R’ groups cannot be, or be connected by, heteroatoms (atoms other than carbon and hydrogen).
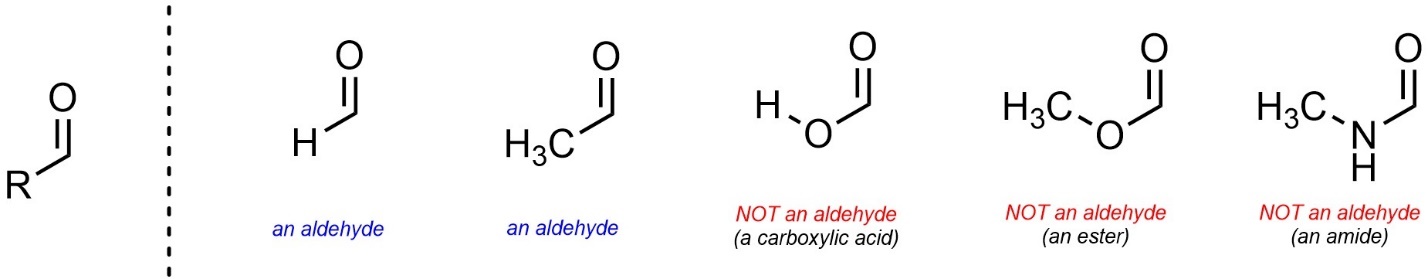
Figure 2.4 – Issues with Atom Choices Using ‘R’ Groups.
There are hundreds of specifically named functional groups. For an introductory text only the most common ones will be important. It is possible that others may be encountered in assignments, labs, discussions, etc. In these cases, searching for the functional group using a resource such as Wikipedia will be a straightforward way of understanding what is being discussed.
2.2.1. Functional Groups Containing Only Carbons (and Hydrogens)
Functional groups containing only carbons and hydrogens are referred to as hydrocarbons. While some molecules are made from only hydrocarbons, more commonly they form the ‘skeleton’ on which other functional groups are placed.
2.2.1.1. Alkanes
The simplest functional group in organic chemistry (which is often ignored when listing functional groups) is called an alkane, characterized by single bonds between two carbons and between carbon and hydrogen. Alkanes do not have a good generic representation, so examples are more convenient (Figure 2.5).

Figure 2.5 – Examples of Alkanes.
By their nature alkanes do not have π bonds in them. If the molecule also does not contain a ring, then it is said to be saturated. This means it contains the maximum number of hydrogens possible based on the number of carbons in the formula (it is saturated in hydrogens). Having a ring or π bond(s) would decrease the number of hydrogens in the formula (Figure 2.6).
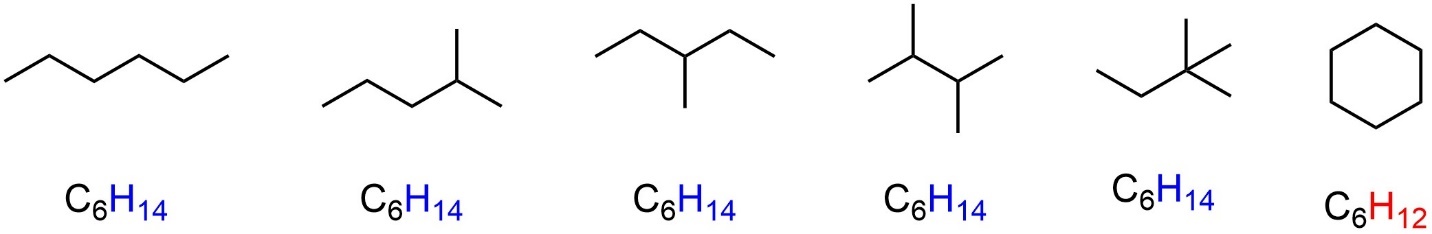
Figure 2.6 – Saturated vs. Unsaturated Alkanes.
2.2.1.2. Alkenes
Alkenes (sometimes called olefins) have carbon-carbon double bonds (Figure 2.7). Alkenes have trigonal planar geometry with sp2 hybridization on both carbons. Because they have a π bond all alkenes are considered unsaturated hydrocarbons.
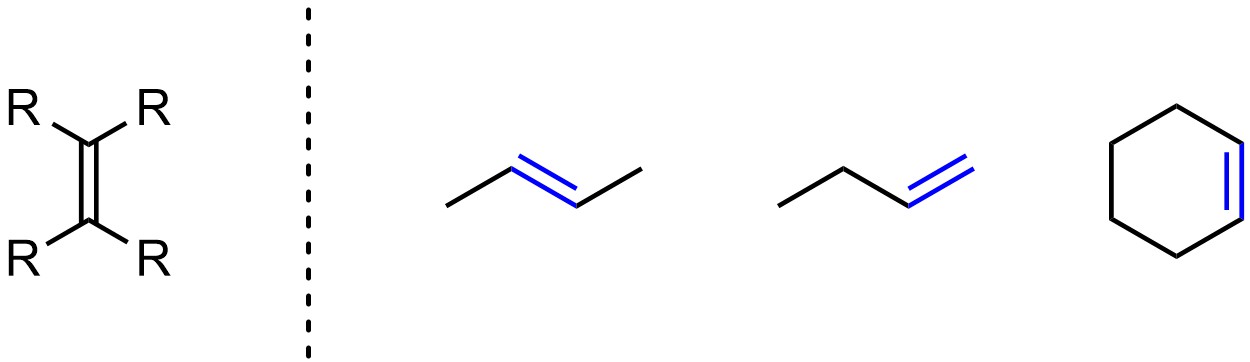
Figure 2.7 – Representation and Examples of Alkenes.
2.2.1.3. Alkynes
Alkynes have carbon-carbon triple bonds (Figure 2.8). Alkynes have linear geometry with sp hybridization on both carbons. Because they have two π bonds all alkynes are considered unsaturated hydrocarbons.
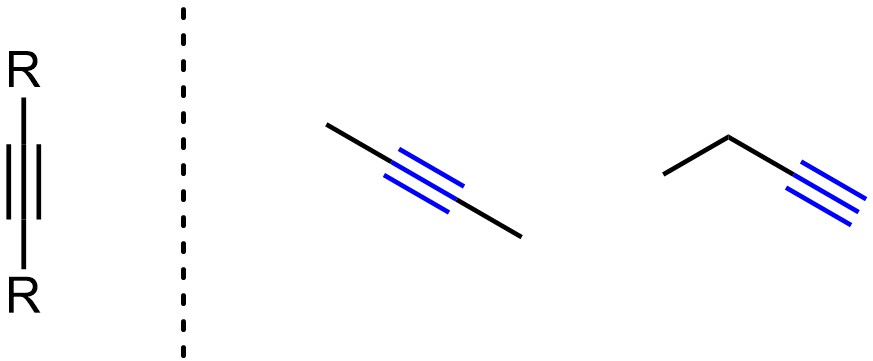
Figure 2.8 – Representation and Examples of Alkynes.
2.2.1.4. Simple Aromatic Compounds
Aromaticity is a complex concept with several specific criteria needed to qualify for the name (see Chapter 9). As a simplification, aromatic hydrocarbons can be thought of as cyclic structures (rings) where an alternating pattern of single and double bonds connect the carbons (Figure 2.9). It is important to remember for later that this explanation is incomplete; even some simple molecules which fit this description may not be aromatic. Understanding why some of these are aromatic and others are not is not important for the discussion of functional groups, and will instead be revisited when discussing aromatic compounds in-depth.
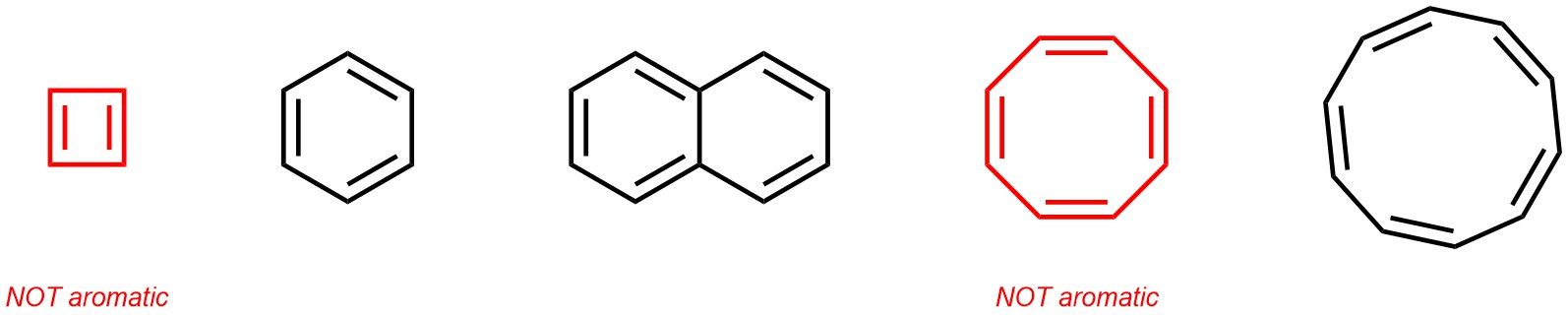
Figure 2.9 – Examples of Aromatic Hydrocarbons.
2.2.2. Functional Groups Containing Carbons and Heteroatoms (and Hydrogens)
As previously mentioned, there are hundreds of specifically named functional groups. Only the most common groups will be discussed in this section.
2.2.2.1. Primary, Secondary, and Tertiary Alcohols
Alcohols are functional groups where a carbon is connected to an oxygen, which is then connected to a hydrogen (Figure 2.10). Alcohols are subdivided into primary, secondary, and tertiary alcohols. The distinction is in the number of carbons attached to the main carbon of the alcohol. A primary alcohol has 1 carbon attached to that carbon, a secondary has 2, and a tertiary has 3. Technically methanol, which has 0 carbons attached to that carbon, is also classed as a primary alcohol.
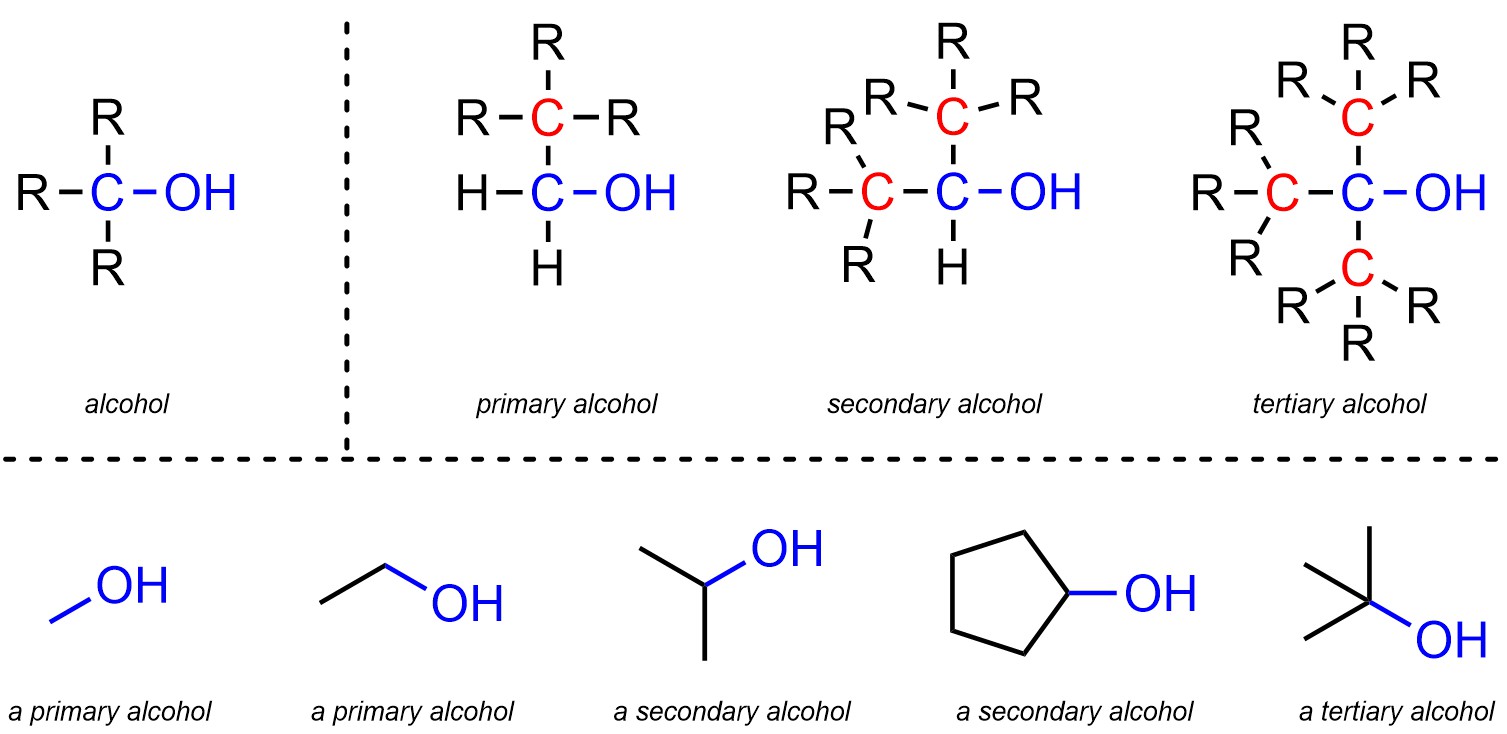
Figure 2.10 – Representation and Examples of Alcohols.
Alcohols are subdivided like this due to the types of reactions they can undergo. For example, while all alcohols have generally the same chemical reactivity a primary alcohol can undergo some reactions that a tertiary alcohol cannot, and vice versa.
2.2.2.2. Ethers
Ethers are functional groups where a carbon is connected to an oxygen, which is then connected to another carbon (Figure 2.11). Ethers are not generally subdivided into separate classes.
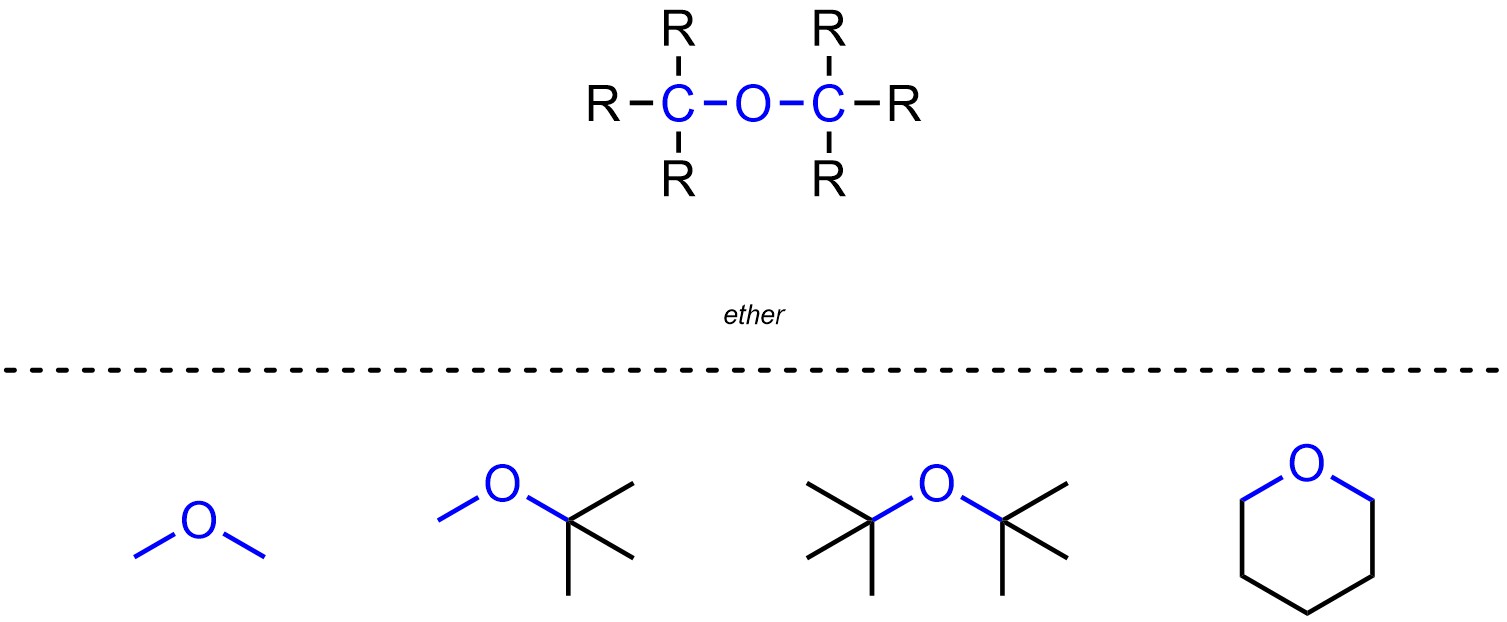
Figure 2.11 – Representation and Examples of Ethers.
2.2.2.3. Aldehydes and Ketones
Aldehydes are functional groups where a carbon is connected to an oxygen through a double bond and also connected to a hydrogen (Figure 2.12). Aldehydes are not generally subdivided into separate classes. Remember that for Line-Angle structures hydrogens attached to carbons are not explicitly shown.
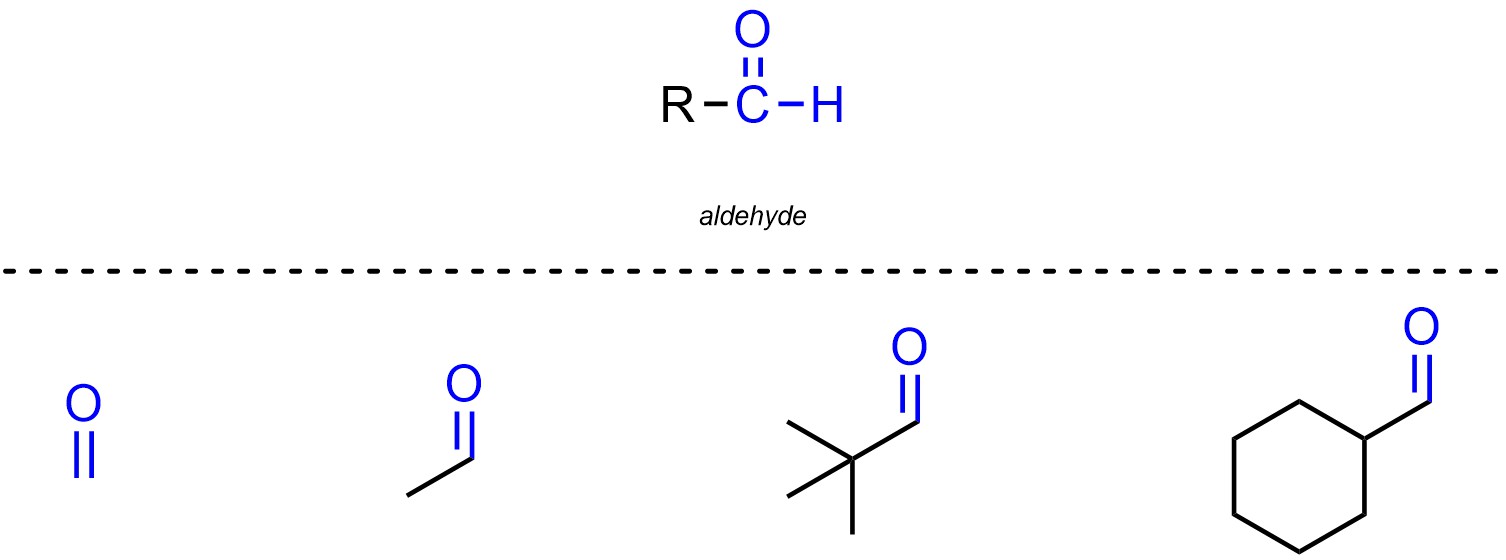
Figure 2.12 – Representation and Examples of Aldehydes.
Ketones are functional groups where a carbon is connected to an oxygen through a double bond and also connected to two other carbons (Figure 2.13). Ketones are not generally subdivided into separate classes.
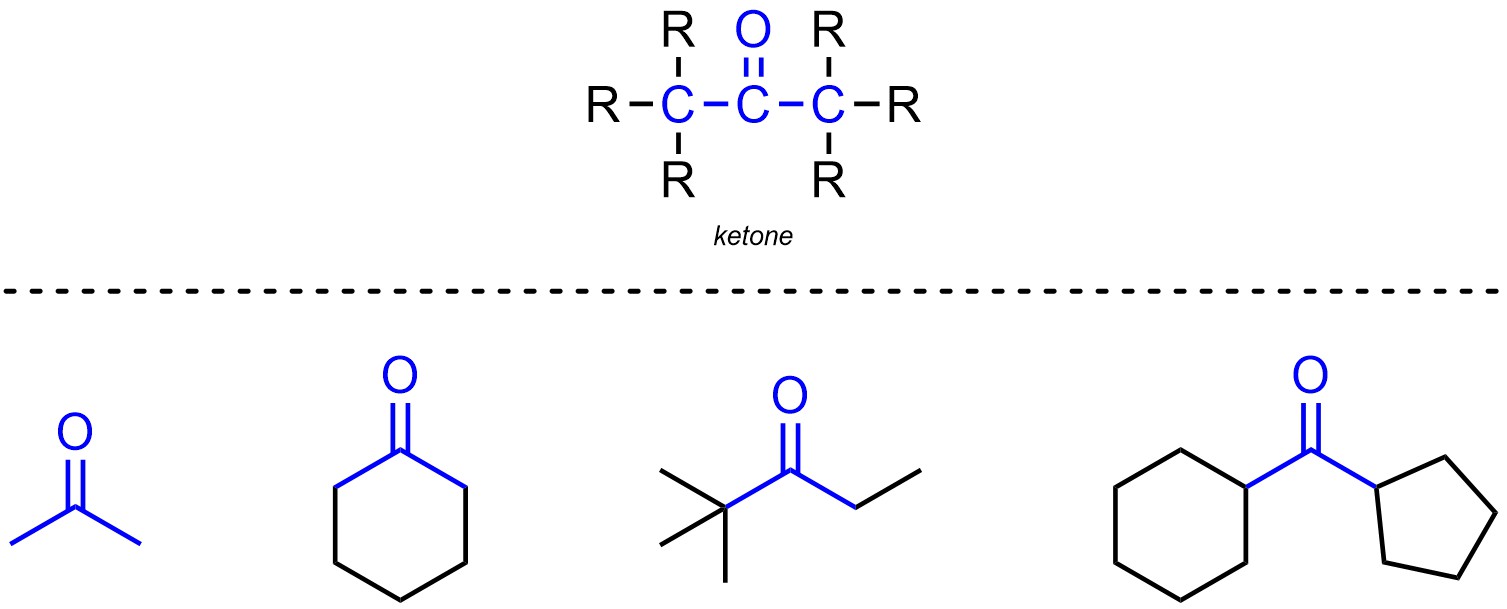
Figure 2.13 – Representation and Examples of Ketones.
2.2.2.4. Carboxylic Acids, Esters, and Acid Halides
Carboxylic acids are functional groups where a carbon is connected to an oxygen through a double bond, then also connected to another oxygen which is connected to a hydrogen (Figure 2.14). Carboxylic acids are not generally subdivided into separate classes.
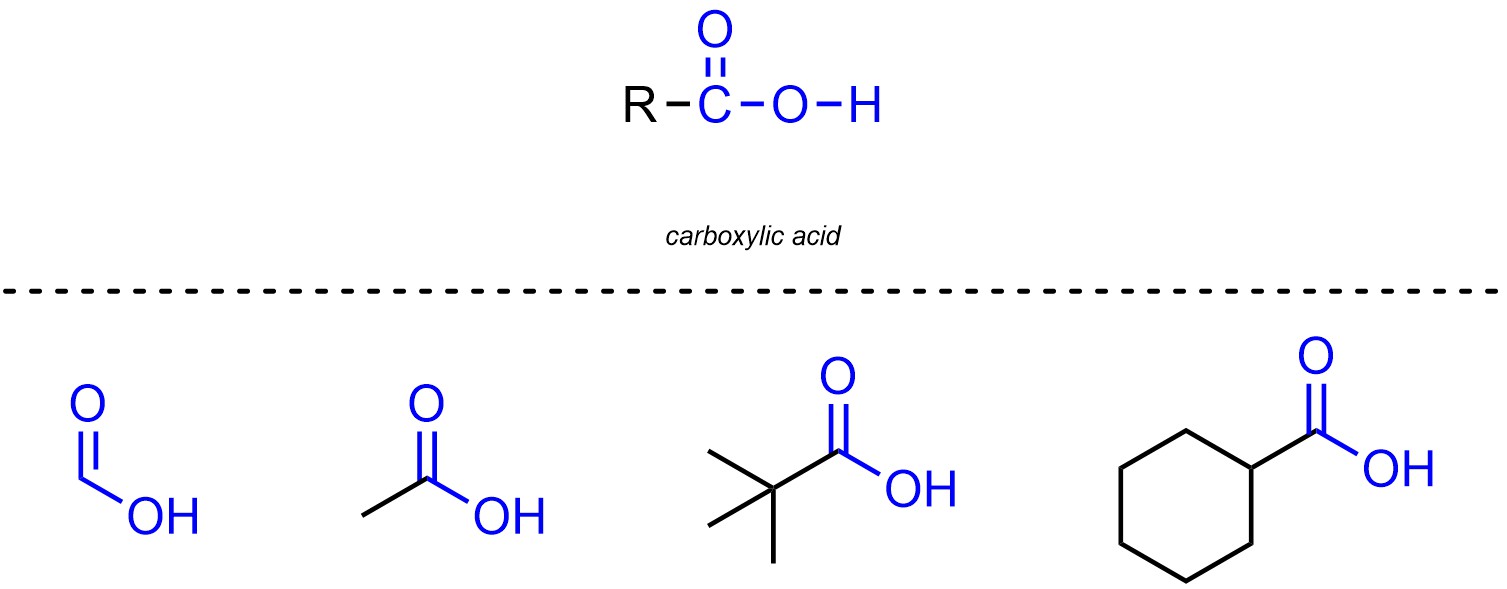
Figure 2.14 – Representation and Examples of Carboxylic Acids.
Esters are functional groups where a carbon is connected to an oxygen through a double bond, then also connected to another oxygen which is connected to a different carbon (Figure 2.15). Esters are not generally subdivided into separate classes.
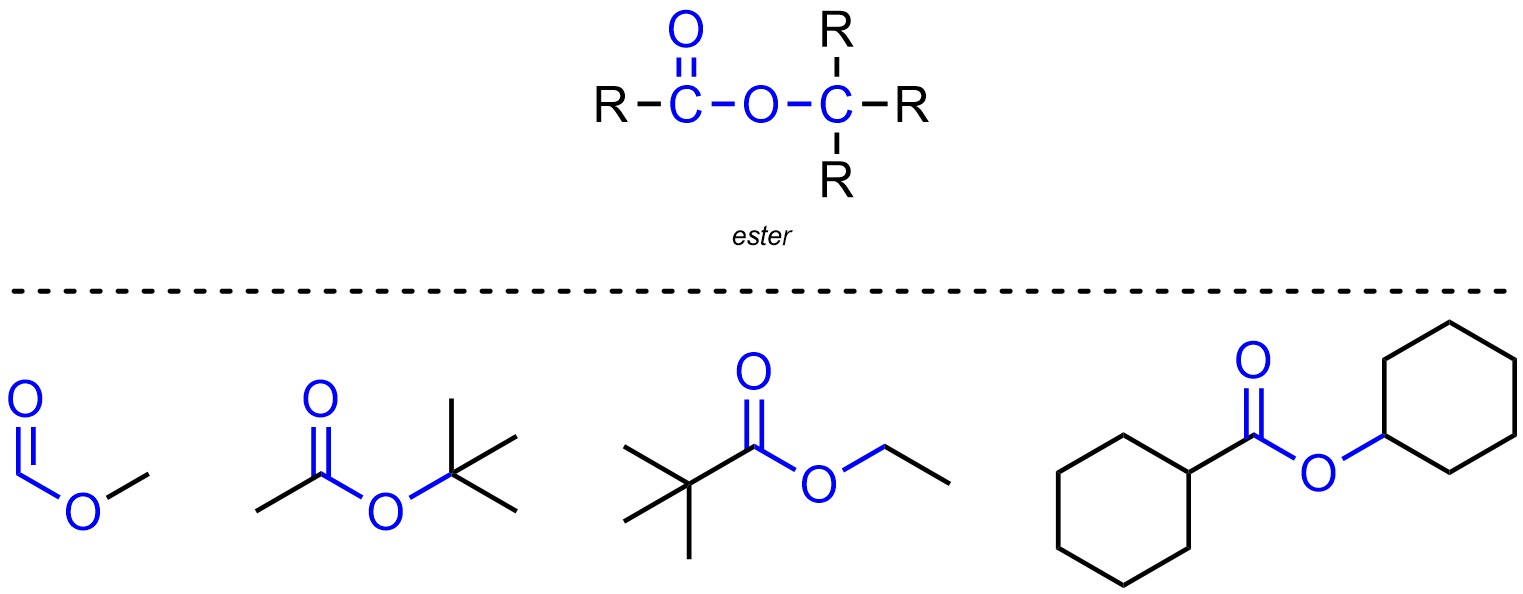
Figure 2.15 – Representation and Examples of Esters.
Acid halides (sometimes called acyl halides) are functional groups where a carbon is connected to an oxygen through a double bond and also connected to a halogen (Figure 2.16). Acid halides are subdivided into separate classes based on the halogen. Although other halogens are possible, the overwhelming majority of acid halides are acid chlorides and acid bromides.
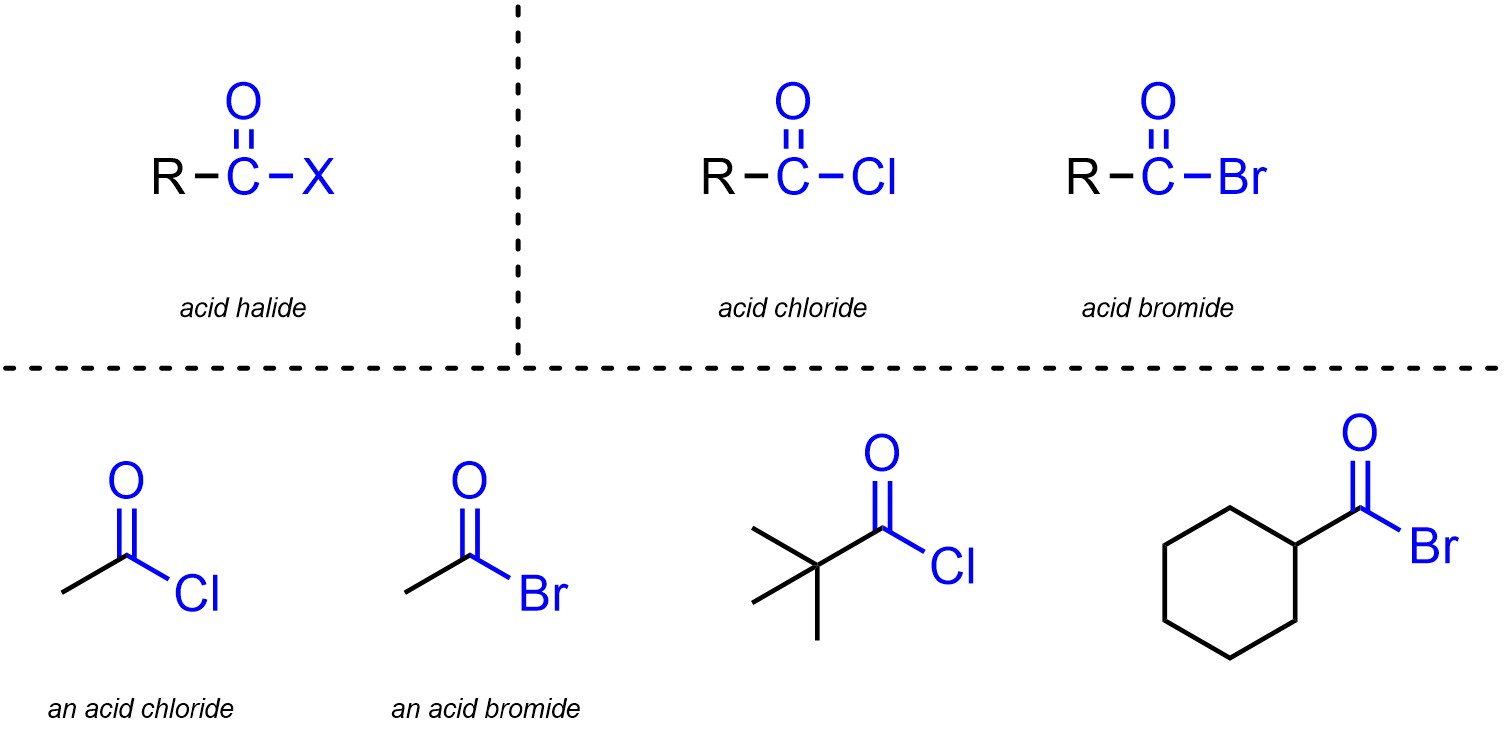
Figure 2.16 – Representation and Examples of Acid Halides.
2.2.2.5. Hydrates, Hemiacetals, and Acetals
Hydrates are functional groups where a carbon is connected to two oxygens, which are both connected hydrogens (Figure 2.17). Hydrates are not generally subdivided into separate classes.
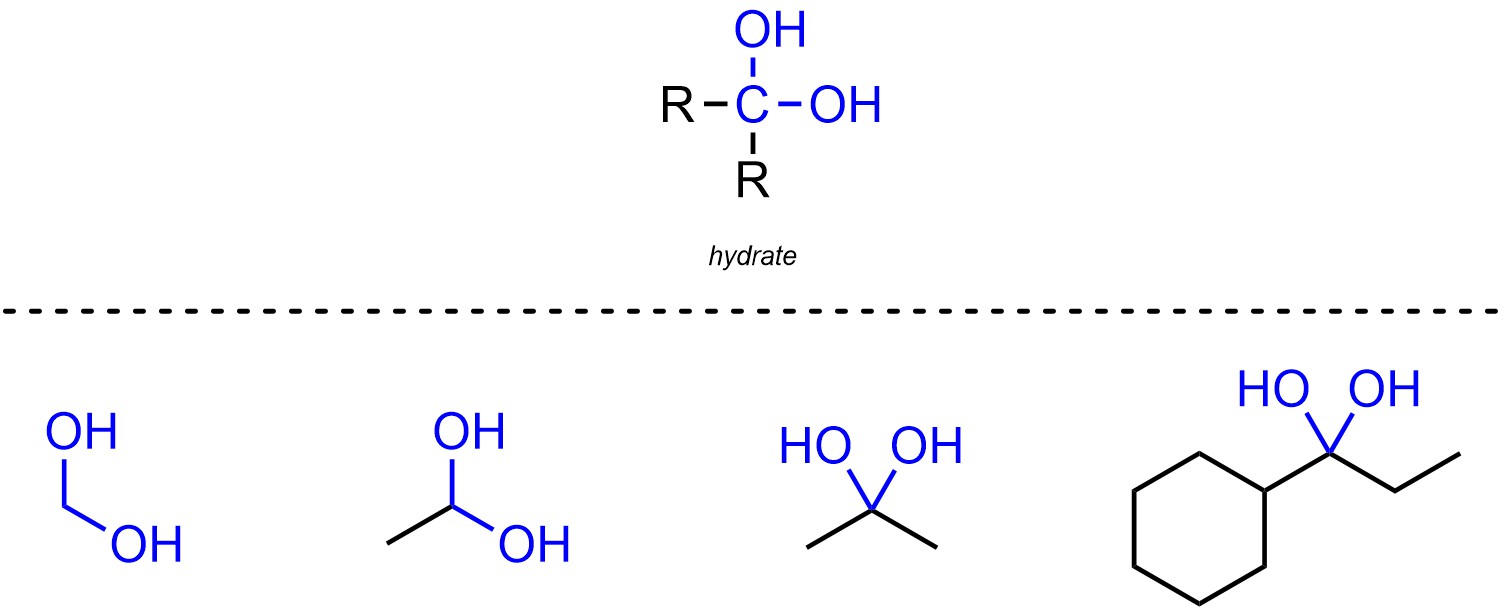
Figure 2.17 – Representation and Examples of Hydrates.
Hemiacetals are functional groups where a carbon is connected to two oxygens, one of which is connected to a hydrogen and the other of which is connected to another carbon (Figure 2.18). Hemiacetals are not generally subdivided into separate classes.
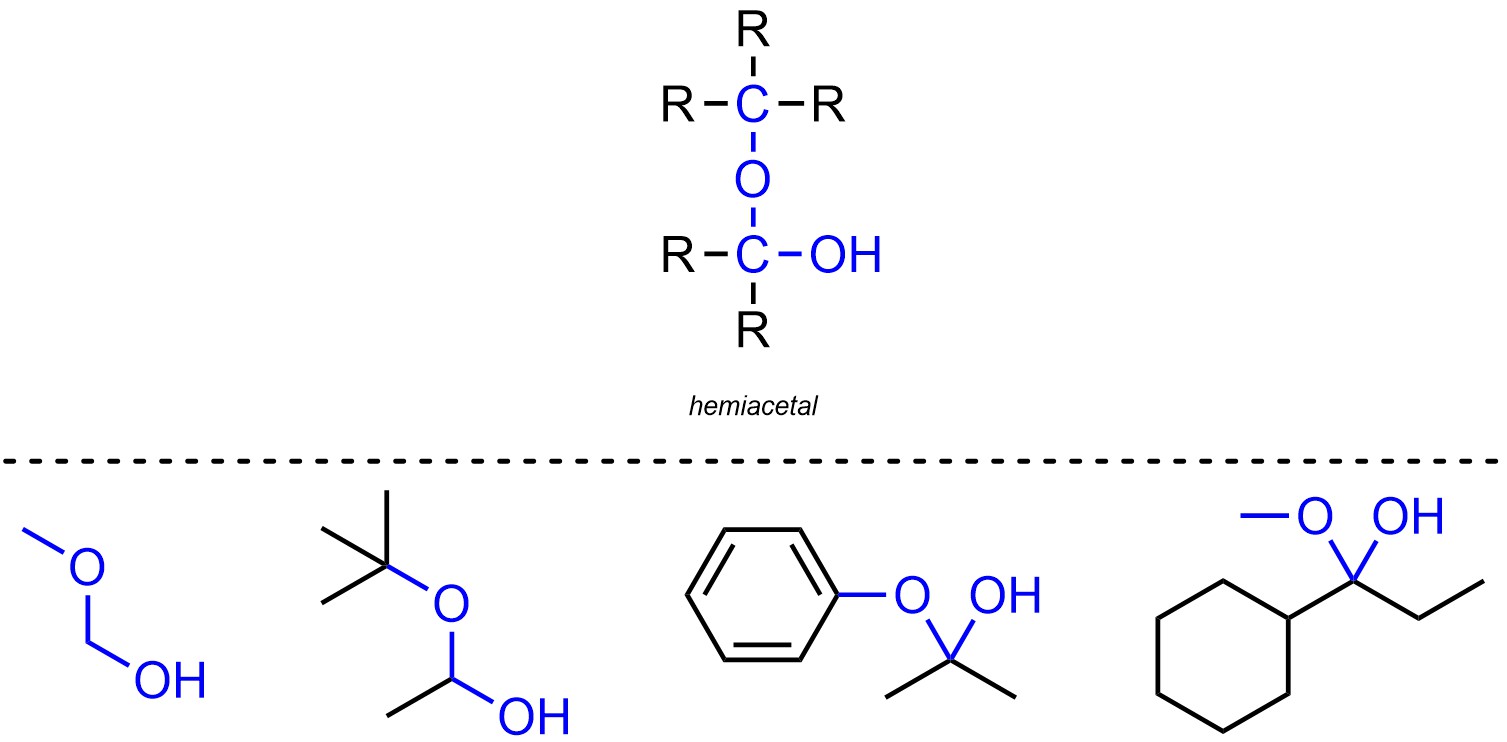
Figure 2.18 – Representation and Examples of Hemiacetals.
Acetals are functional groups where a carbon is connected to two oxygens, which are both connected to other carbons (Figure 2.19). Acetals are not generally subdivided into separate classes.
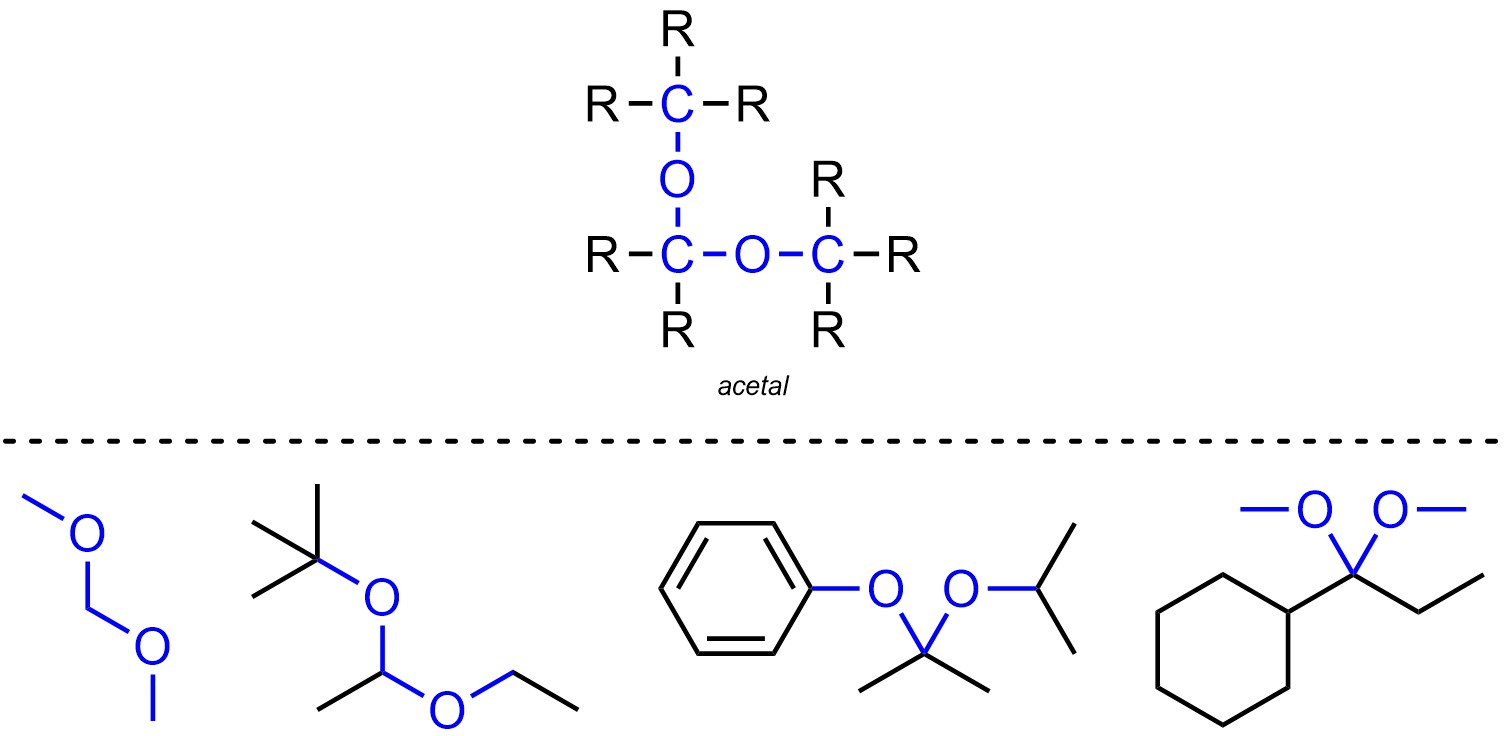
Figure 2.19 – Representation and Examples of Acetals.
2.2.2.6. Amides
Amides are functional groups where a carbon is connected to an oxygen through a double bond and also connected to a nitrogen (Figure 2.20). Amides are often subdivided into primary, secondary, and tertiary amides. However, the specific criteria for this subdivision are not widely agreed on. As such, subdivision of amides is not required in this text.
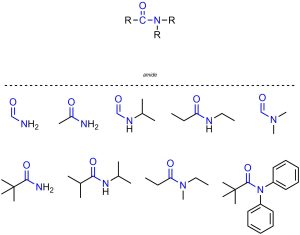
Figure 2.20 – Representation and Examples of Amides.
2.2.2.7. Primary, Secondary, and Tertiary Amines
Amines are functional groups where a nitrogen is connected to carbons and/or hydrogens (Figure 2.21). Amines are subdivided into primary, secondary, and tertiary amines. The distinction is in the number of carbons attached to the nitrogen. A primary amine has 1 carbon attached to the nitrogen, a secondary has 2, and a tertiary has 3. Technically ammonia, which has 0 carbons attached to nitrogen, is also classed as a primary amine.
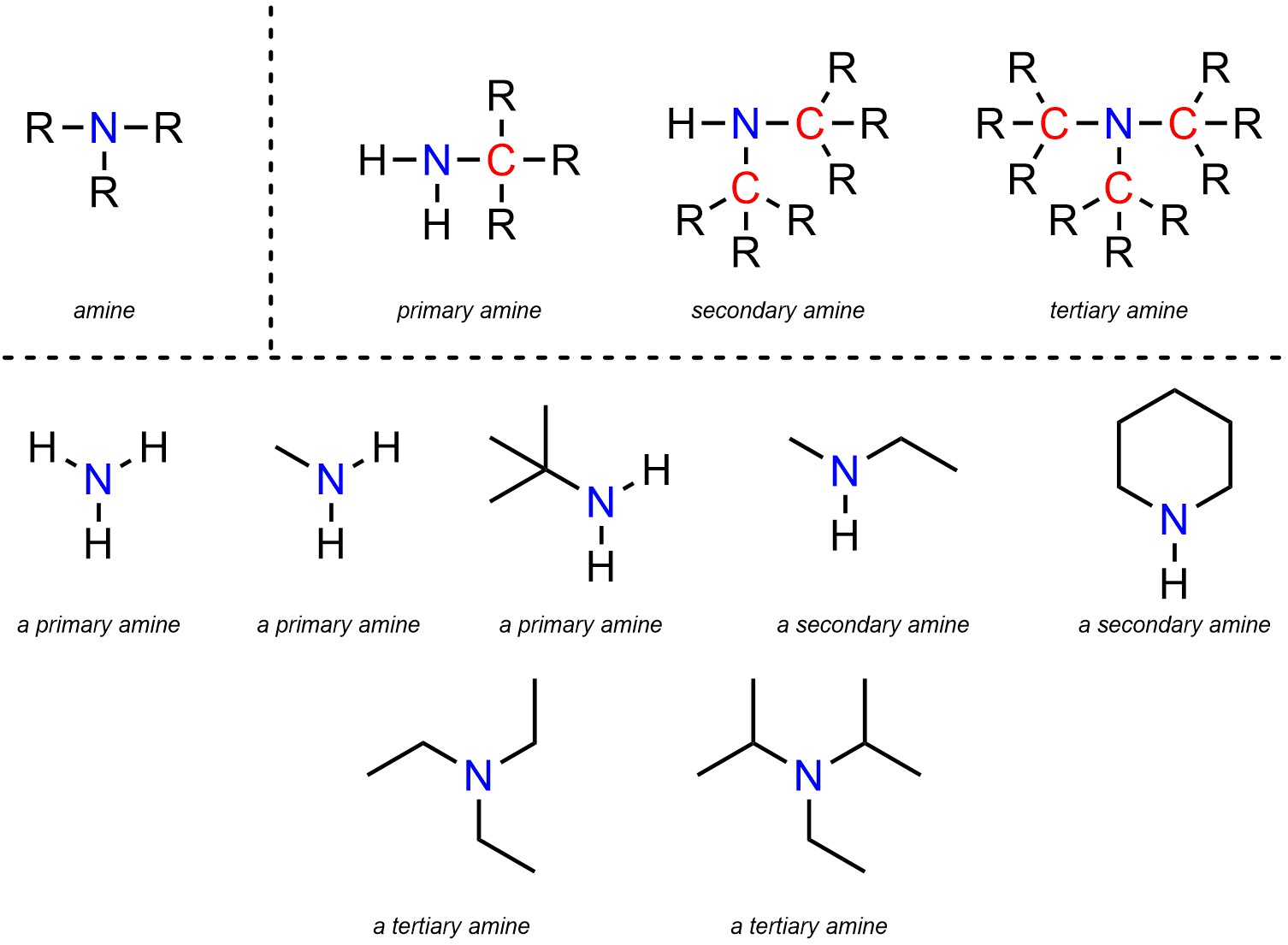
Figure 2.21 – Representation and Examples of Amines.
Amines are subdivided like this due to the types of reactions they can undergo. For example, while all amines have generally the same chemical reactivity a primary amine can do some reactions that a tertiary amine cannot, and vice versa.
2.2.2.8. Nitro and Nitriles
Two other nitrogen-containing functional groups are common but not easily related to amides or amines.
Nitro is a functional group where a nitrogen is connected to an oxygen through a double bond and also connected to another oxygen (Figure 2.22). While the nitrogen has 4 bonds and is cationic, one of the oxygens has only one bond and is anionic. The overall charge for the functional group is neutral.
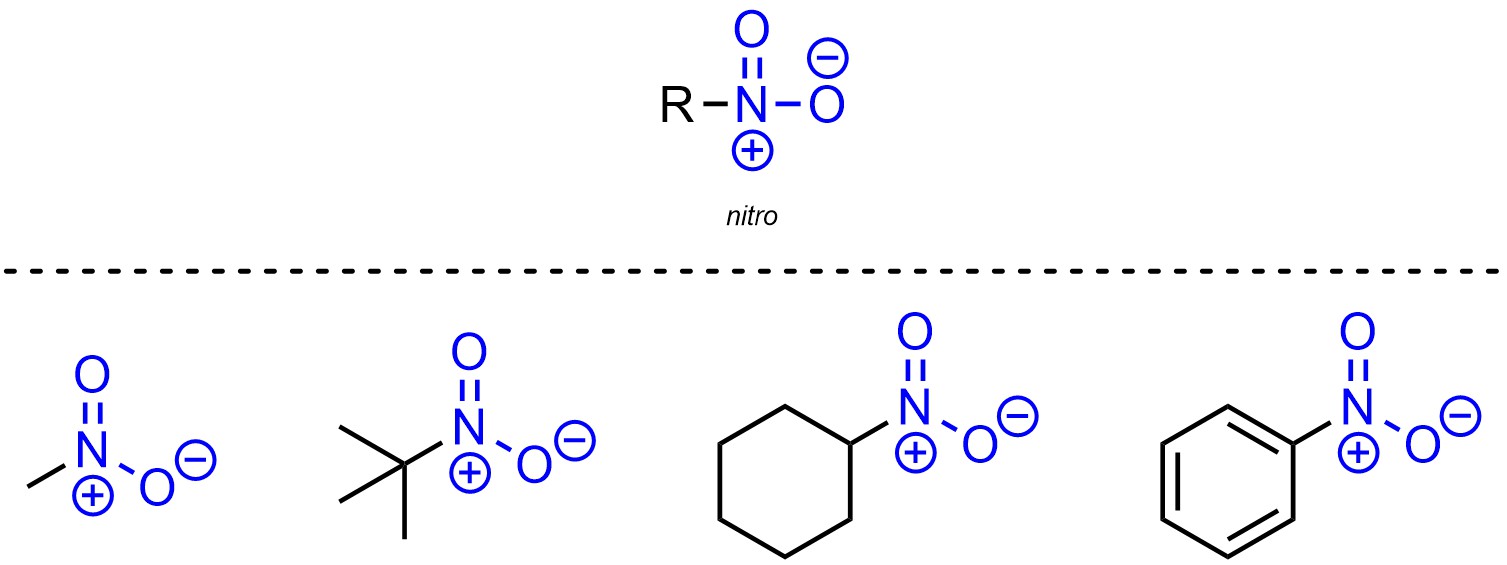
Figure 2.22 – Representation and Examples of Nitro-Containing Molecules.
Nitriles (sometimes called cyanos or cyanides) are functional groups where a carbon is connected to a nitrogen through a triple bond (Figure 2.23).
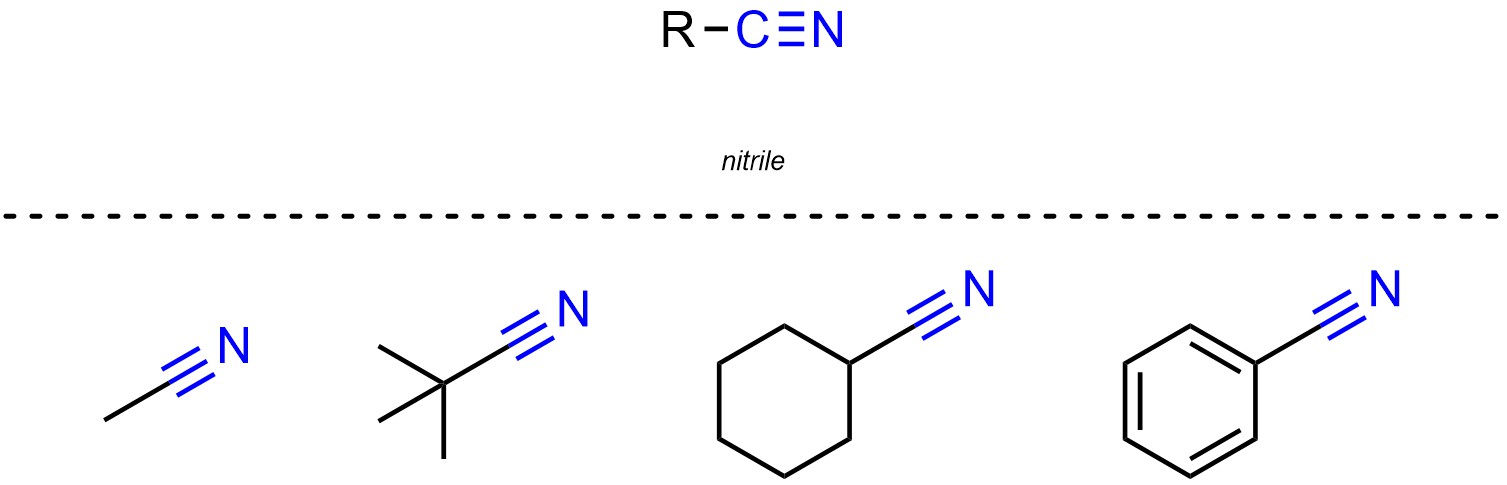
Figure 2.23 – Representation and Examples of Nitriles.
2.2.2.9. Imines
Imines are functional groups where a carbon is connected to a nitrogen through a double bond (Figure 2.24). Imines are simply the nitrogen equivalent of aldehydes and ketones, though they are not normally subdivided in that way.
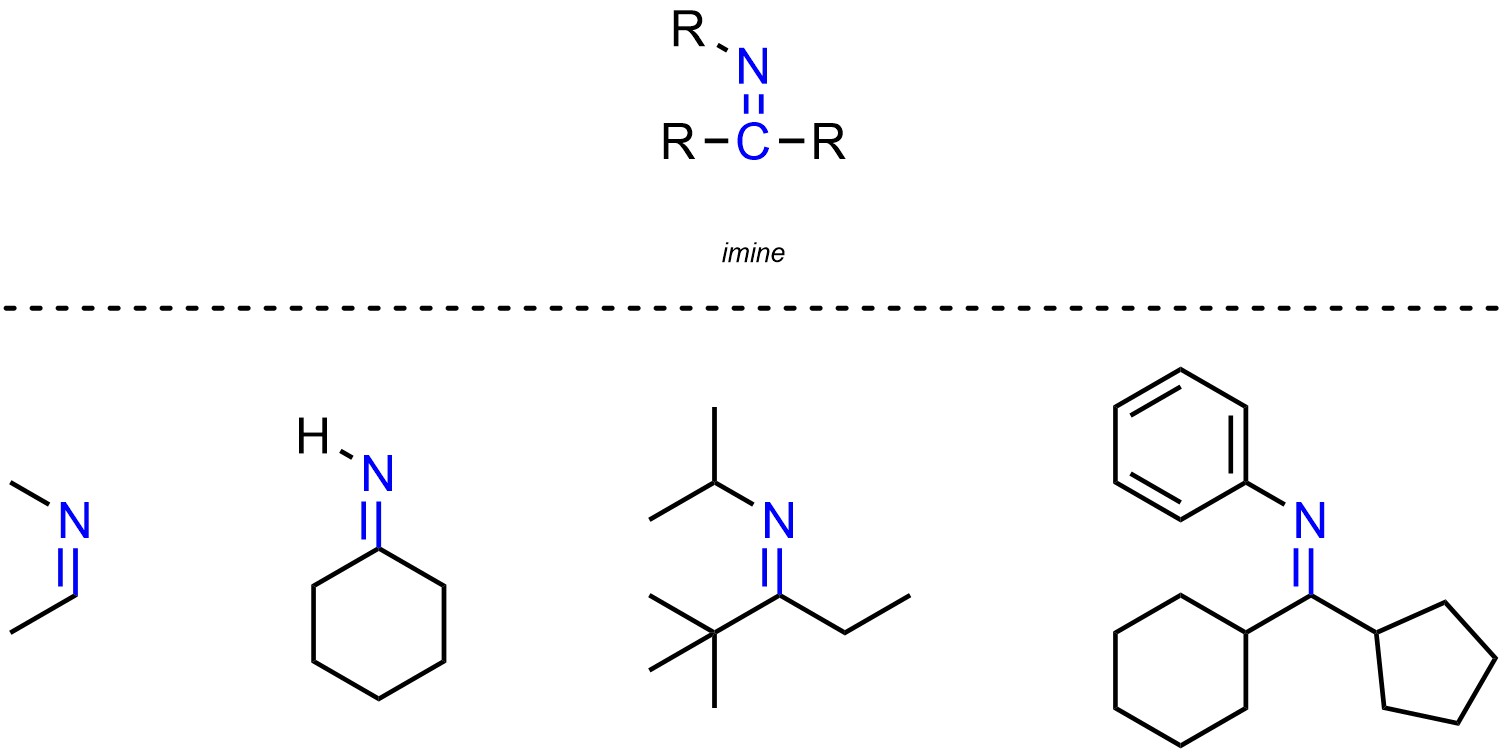
Figure 2.24 – Representation and Examples of Imines.
2.2.2.10. Halides
Halides are functional groups where a carbon is connected to a halogen (Figure 2.25). Halides may be subdivided into separate classes based on which halogen is present.
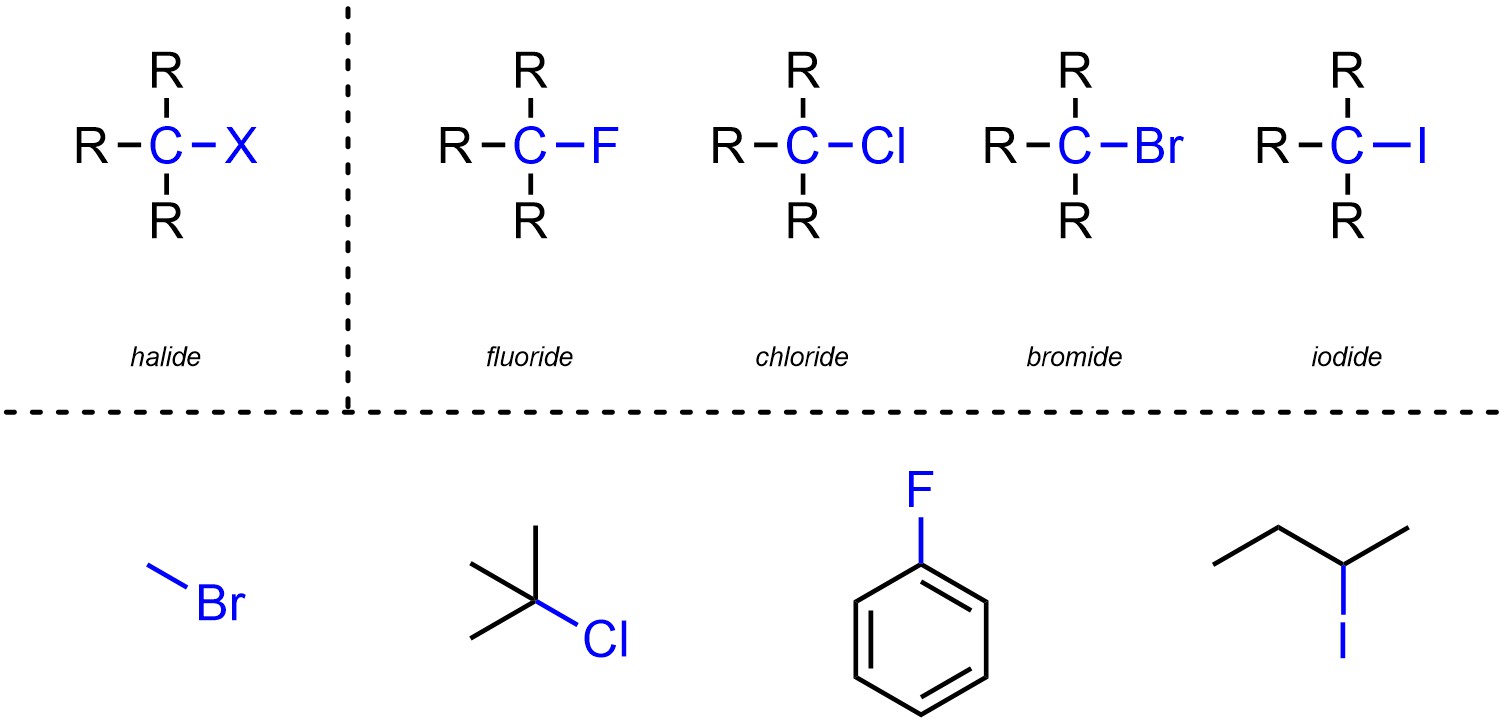
Figure 2.25 – Representation and Examples of Halides.
2.2.2.11. Thio-Equivalents
Though not common in this text, a number of functional groups are simply the sulfur-analogues of common oxygen-containing functional groups. While many of these have their own names, a more common approach is to simply use the prefix “thio” with the oxygen-containing name (Figure 2.26).
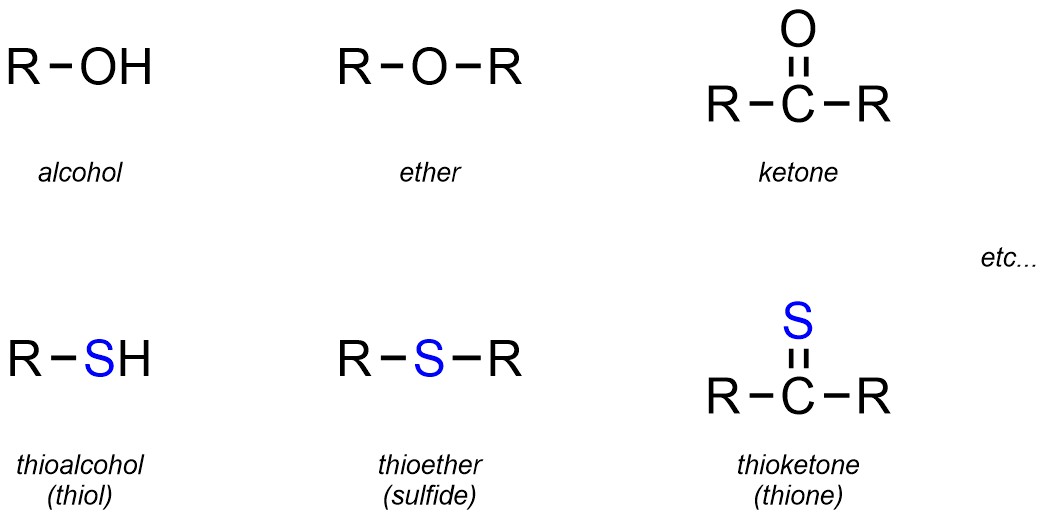
Figure 2.26 – Representation and Examples of Thio-Equivalents of Oxygen-Containing Functional Groups.
For these groups it is important to remember that, unlike C-O and O-H bonds, C-S and S-H bonds are NOT polar (see Figure 1.14). This means that, despite their apparent similarity to the oxygen-containing equivalents, these groups have significantly different reactivities.
2.2.2.12. Extra: Phosphates, Phosphites, Hypophosphites, Phosphonates, and Phosphinates
Though not common in this text, a number of functional groups are based on the oxidation-state of phosphorous. These groups have significant biological relevance but are not commonly encountered in traditional organic chemistry. A number of related functional groups exist, but most can be approximated by adding “ester” to the name if the ‘R’ groups on oxygen contain carbon (Figure 2.27). These groups will not feature in questions or exams but seeing them at this stage may help with context when discussing bioorganic chemistry.
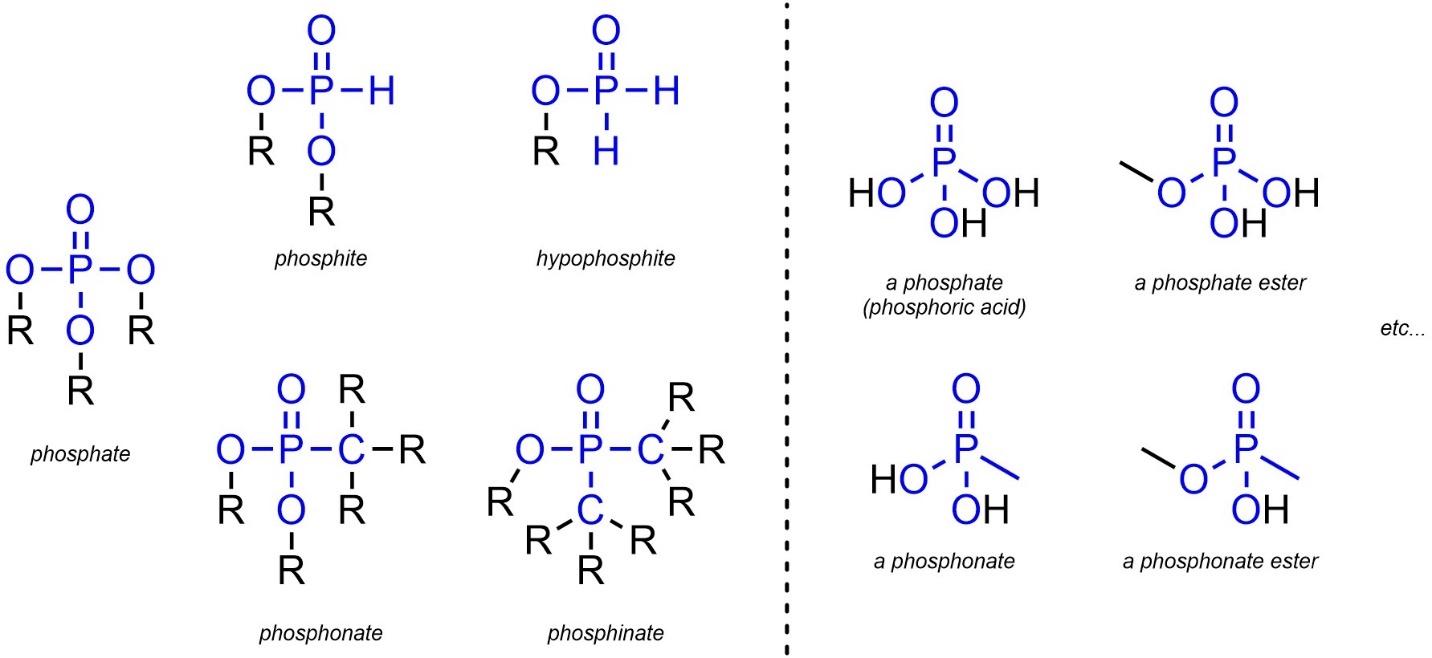
Figure 2.27 – Representations and Examples of Phosphorous-Containing Functional Groups.
2.2.2.13. The Carbonyl Group – An Important Part of Functional Groups
When reviewing the functional groups above you may have noticed that a carbon double bonded to an oxygen (with two other groups on the carbon) was present in many functional groups (Figure 2.28). This arrangement of atoms is so common that it has a special name: a carbonyl.
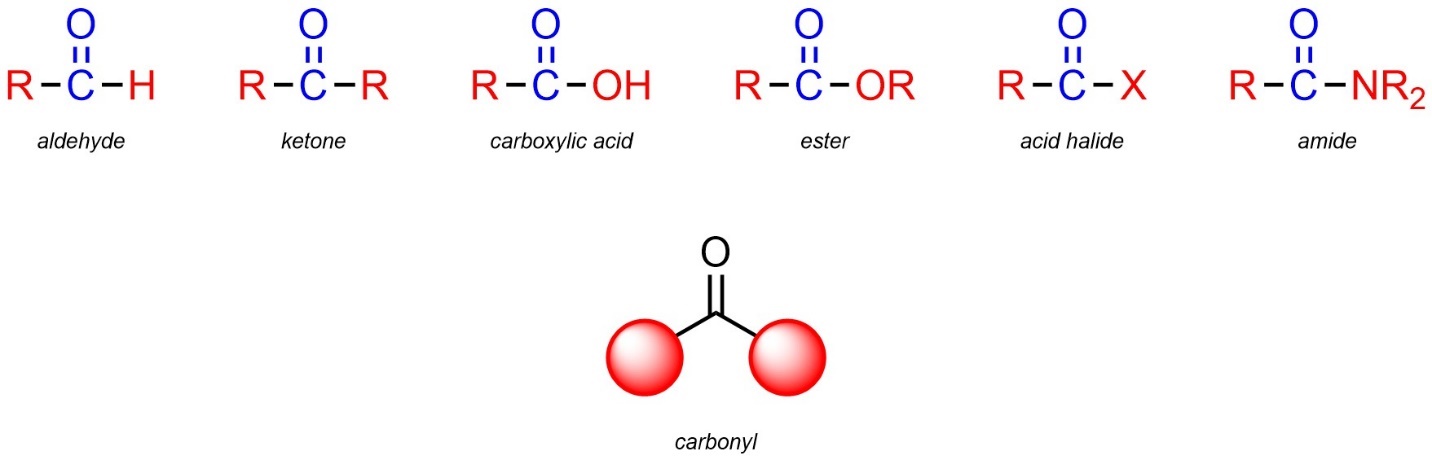
Figure 2.28 – Examples of Carbonyls in Functional Groups.
Carbonyls are not functional groups on their own. However, they are part of many functional groups. Because this feature is common to so many groups, it imparts a similar type of reactivity to all groups with it (see Section 7.3). Later, when these reactions are being discussed in detail, the carbonyl group will be mentioned as a proxy for listing every functional group that contains it.
2.2.3. Summary of Functional Groups
The previous sections seem to list a large number of varying functional groups for memorization. In fact, most of these groups are VERY similar to each other and differ only by an element or two. Ignoring the various sub-classes, it is often helpful to view functional groups by their similarities to each other (Figure 2.29). Since structure dictates reactivity, recognizing which functional groups are related (and how they are similar/different) can be very helpful for understanding patterns in chemical reactivity.
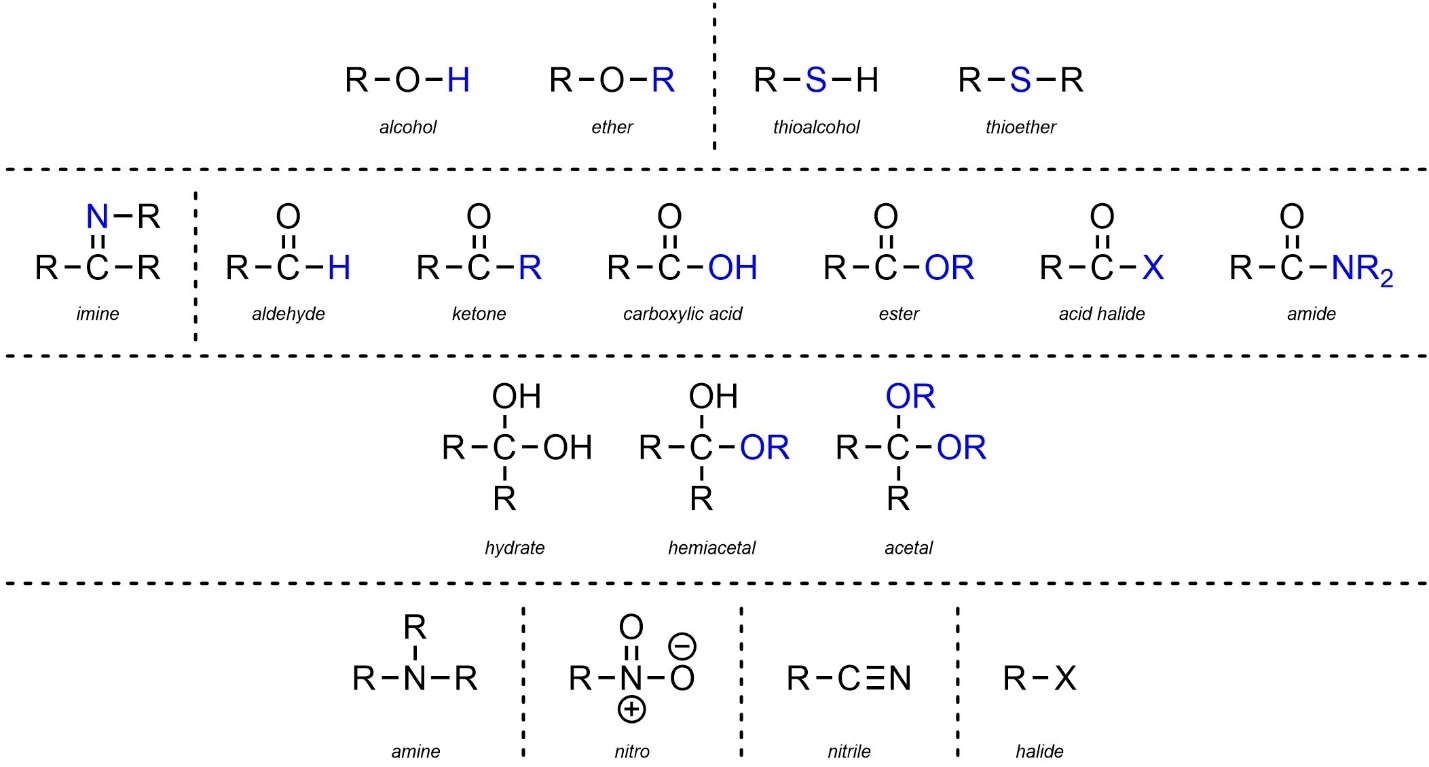
Figure 2.29 – Summary and Highlighted Comparisons of Functional Groups.
A common task in organic chemistry is to identify functional groups in complex molecules. This helps pinpoint areas where reactions may be occurring, what reactions may be possible, etc. One should be able to look at any molecule and be able to quickly identify the various functional groups in it (Figure 2.30).
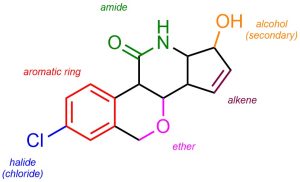
Figure 2.30 – Example of a Complex Molecule with Labelled Functional Groups.

