9.5. Nomenclature
Aromatic and heteroaromatic rings may be named systematically using the IUPAC rules. Often, they are instead named using relative descriptors or trivial names.
9.5.1. Trivial Names of Common Aromatic and Heteroaromatic Compounds
Most aromatic compounds were identified and characterized before the IUPAC system was developed. As a result, the use of trivial names for aromatic compounds remains commonplace and memorizing the more common ones is required for communication (see Section 9.5.2.). There are hundreds of specifically named aromatic rings. For an introductory text only the most common ones will be important (Figure 9.18). Others may be encountered in assignments, labs, discussions, etc. In these cases, searching for the ring using a resource such as Wikipedia will be a straightforward way of understanding what is being discussed.
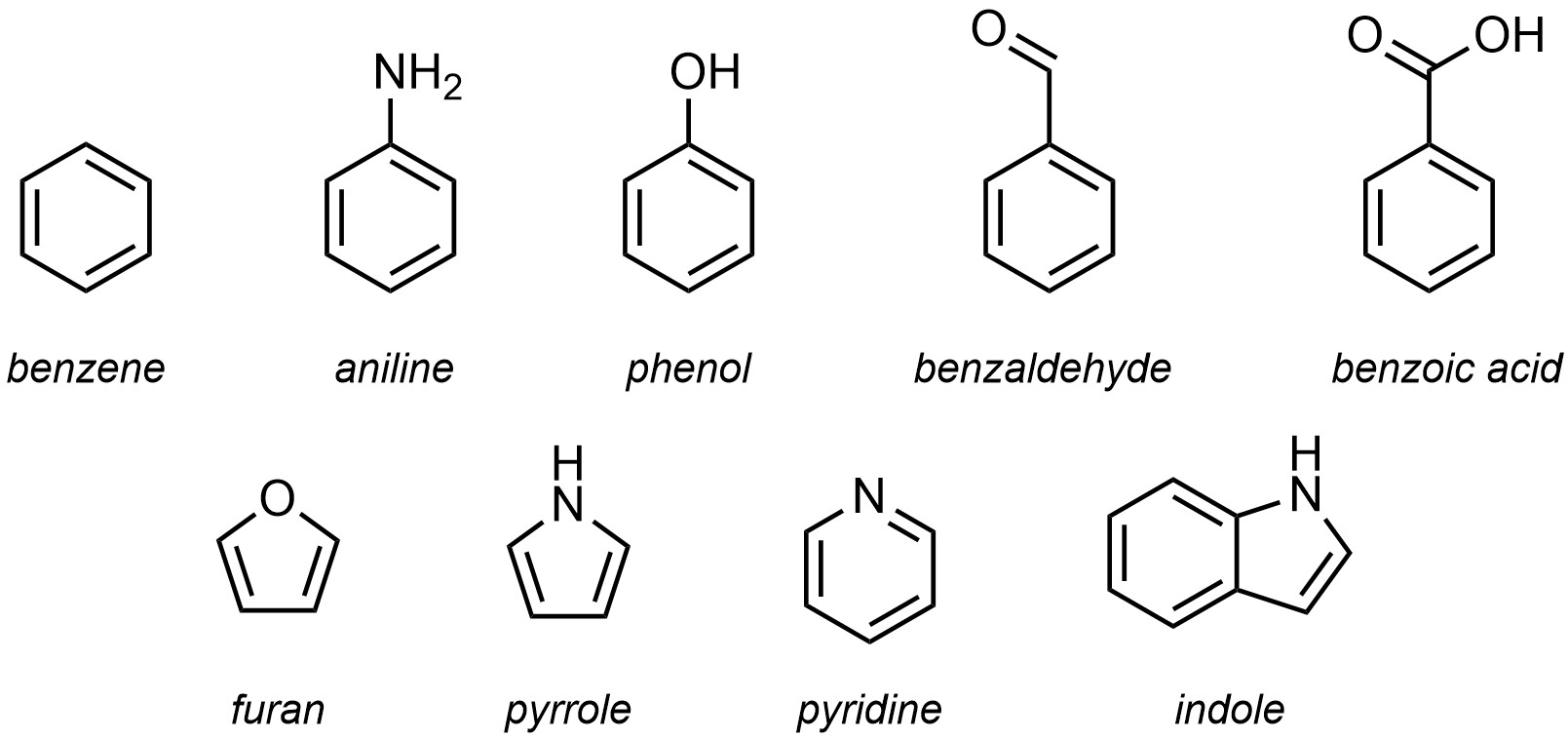
Figure 9.18 – Trivial Names for Common Aromatic and Heteroaromatic Rings.
9.5.2. IUPAC Numbering
Most aromatic compounds were identified and characterized before the IUPAC system was developed. As a result, the use of trivial names for aromatic compounds was already prevalent to such an extent that they became part of the formal system. Simple aromatic rings are often named using the trivial name of the compound that contains as many of its functional groups as possible for the root/suffix (see Section 9.5.2.1; Figure 9.19). Specific suffix use is often not required as the trivial names account for many of the common functional groups. Prefixes for additional substituents are prepended as usual.
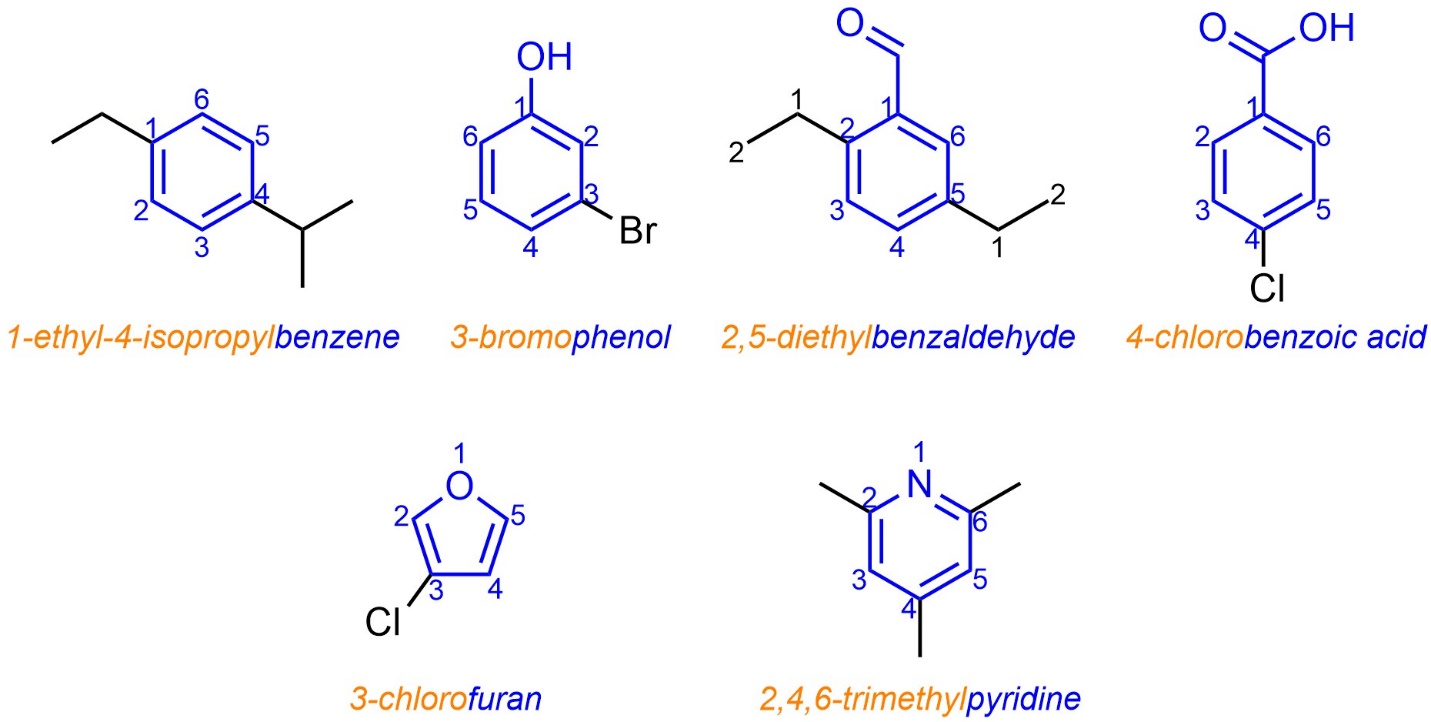
Figure 9.19 – Examples of IUPAC-Named Aromatic Molecules Using Trivial Names.
9.5.2.1. Greek Descriptors: ortho (o) vs. meta (m) vs. para (p)
A set of Greek relative descriptors is commonly used alongside or in place of the IUPAC numbering system (Figure 9.20). Ortho (abbreviated as an italicized o) is used to describe a 1,2 relative relationship. Meta (abbreviated as an italicized m) is used to describe a 1,3 relative relationship. Para (abbreviated as an italicized p) is used to describe a 1,4 relative relationship.
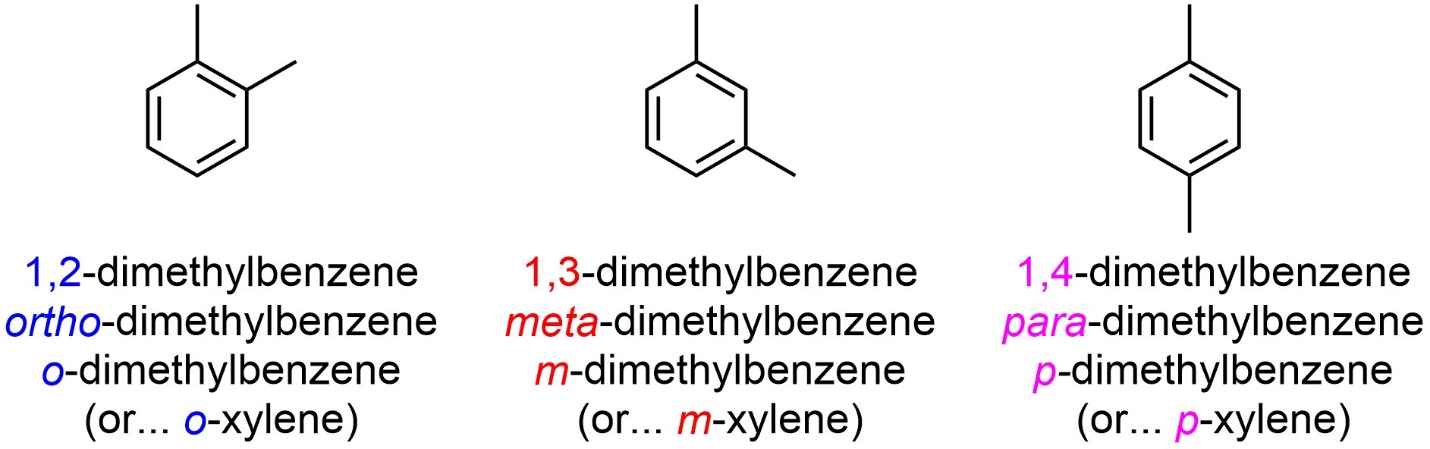
Figure 9.20 – Simple Examples of the Use of the Ortho/Meta/Para System.
The use of IUPAC numbering is generally preferred for formal naming. However, ortho/meta/para descriptors are exceptionally useful for discussion of chemical reactions of aromatic rings (see Section 10). Both systems must be understood.
9.5.2.2. Limitations of the o/m/p System
For naming purposes, the ortho/meta/para descriptors are only used for six-membered carbocyclic aromatic rings (Figure 9.21). Because they are relative, heteroaromatic rings cannot be properly named using these descriptors.
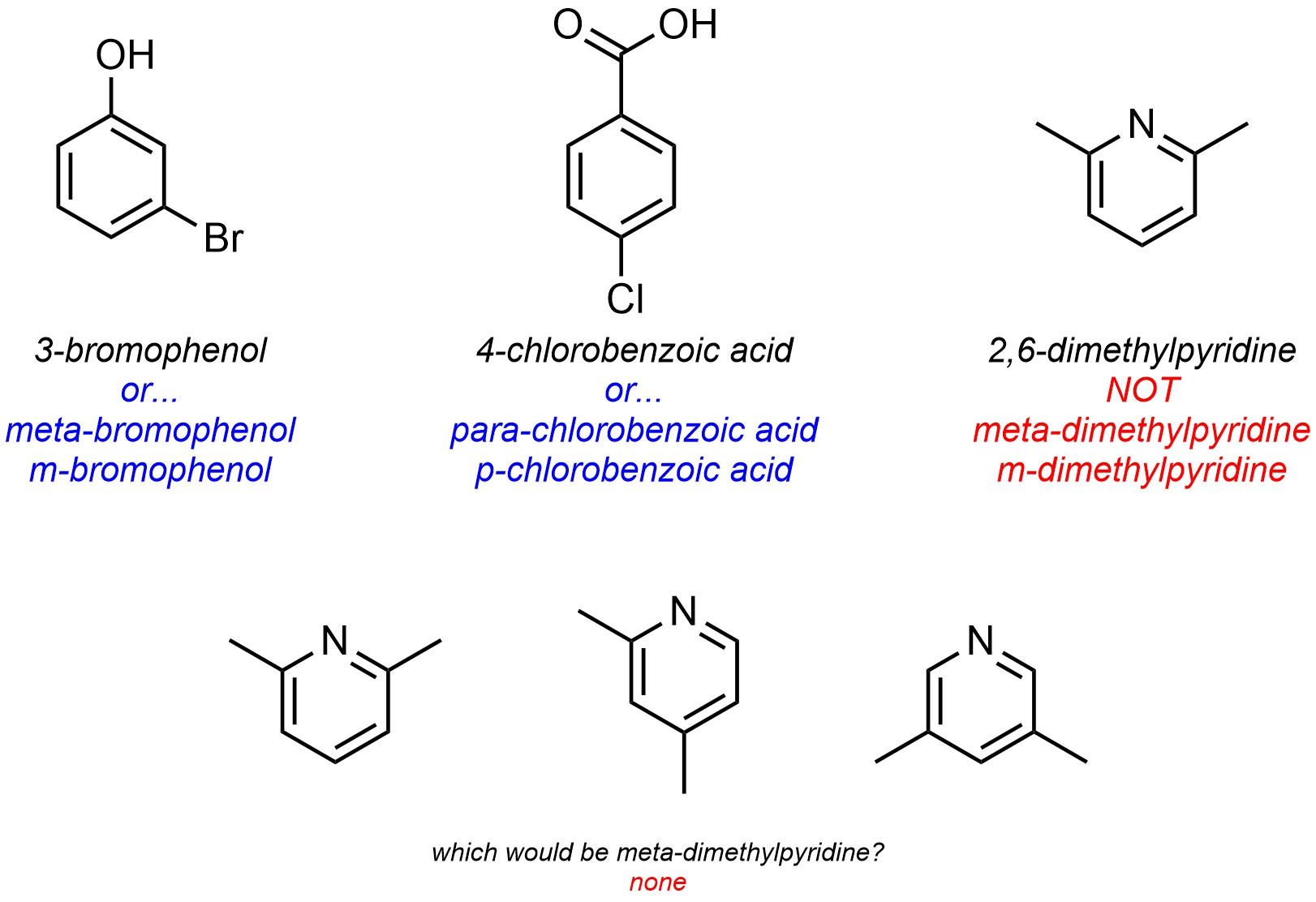
Figure 9.21 – Example of why Greek Relative Descriptors Cannot be Used for Heteroaromatic Rings.
Because these descriptors are relative it is not possible to use them for naming compounds with more than two substituents (Figure 9.22). However, as with other relative descriptors (see Figure 3.19 and/or Section 4.4.3.), it is possible to describe the relative positions of substituents/groups using these terms. This also applies to heteroaromatic systems.
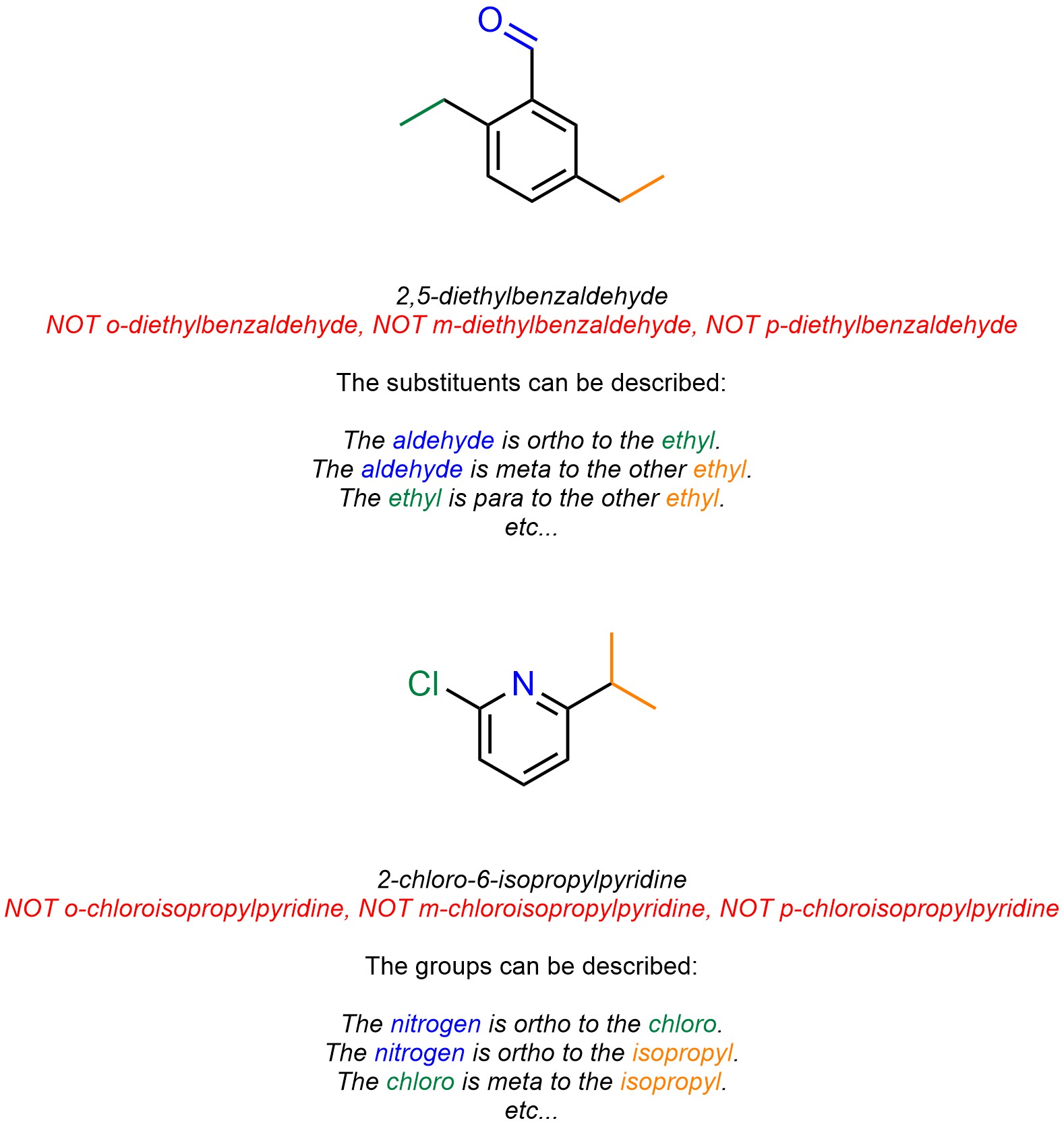
Figure 9.22 – Examples of the Use of Ortho/Meta/Para as Relative Descriptors.

