2.5. Nomenclature
The rules for naming organic molecules are established by the International Union of Pure and Applied Chemistry (IUPAC). Having a standardized set of rules for naming compounds allows chemists to communicate with each other more easily. Using the IUPAC nomenclature system we can derive the name of an organic compound from its structure and draw the structure of a molecule based on its name.
The actual IUPAC guidelines for nomenclature are spread across several volumes and total well over a thousand pages. While this level of detail is necessary to deal with all possible cases, at an introductory level it is excessive. In place of giving a complete guide to nomenclature this text will aim to convey the basics of the system. In order to keep things simple, the exact and accurate rules are not always being communicated. The goal is for readers to be able to name simple compounds and be able to analyze moderately complex names to determine the structure they describe.
2.5.1. Basics of Nomenclature
A compound’s systematic name has three portions: a prefix, which contains details about the substituents on the molecule; a root name, which describes how many carbons are in the longest chain and if/where there are any alkenes or alkynes; a suffix, which contains details about the principal (highest ‘priority’) functional group in the molecule (Figure 2.40). For molecules with stereochemical information (see Chapter 4) this is also included in front of the prefix. For now we will ignore this portion.
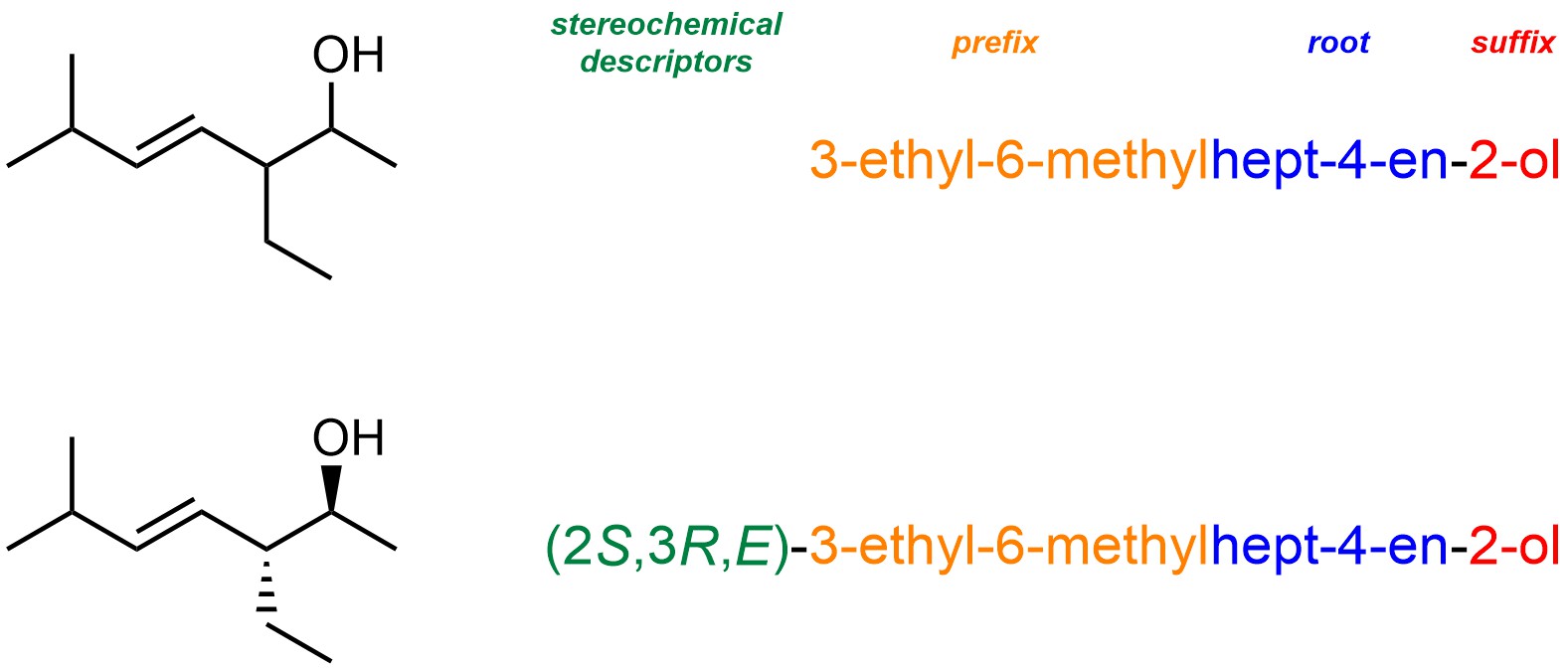
Figure 2.40 – Example of an IUPAC Systematic Name for a Compound.
2.5.2. Simple Alkanes and Root Names
Recall that the simplest functional group, which is often ignored when listing functional groups, is an alkane. While some molecules are made from only alkanes, more commonly they form the ‘skeleton’ on which other functional groups are placed. In molecules where alkanes are the only functional group they become the principal functional group by default. This means that these compounds will have the suffix “ane”, which represents alkane.
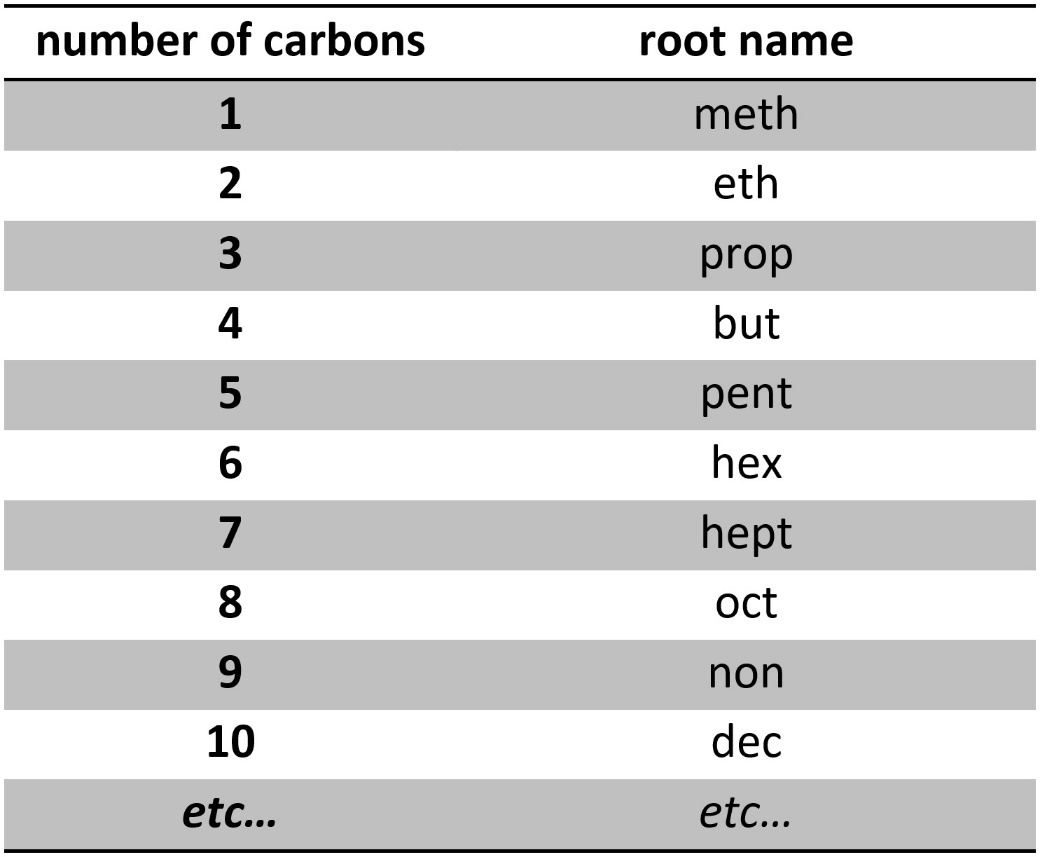
Root names describe the longest contiguous carbon chain in the molecule. Because the overwhelming majority of organic molecules have a carbon ‘skeleton’ these names are universal and appear in almost all systematic names. The root component is a descriptor for the number of carbons in the chain. When the chain is short (1 to 4 carbons) the words are specific to chemistry. However, for longer chains (≥5 carbons) the system defaults to Greek (Table 2.4). Memorizing the root names is an unfortunate necessity for competent communication.
Table 2.4 – Root Names Based on Longest Contiguous Carbon Chain Length.
In simple alkanes there are no substituents, so the prefix component is left blank (Figure 2.41).
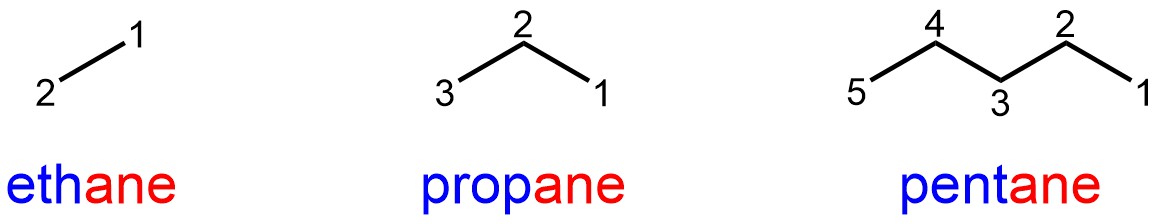
Figure 2.41 – Examples of Named Simple Alkanes.
2.5.3. Branched Alkanes
As alkanes are the simplest functional group, they are also the simplest substituent. The same rules for the suffix and root portions apply, but now there will be a prefix component.
When alkanes are substituents the substituent gets named using the root word appropriate for the number of carbons in the substituent, but with “yl” appended (alkane substituents are often called ‘alkyl’ groups; Table 2.5).
Table 2.5 – Substituent Names for Alkyl Groups Based on Longest Contiguous Carbon Chain Length.
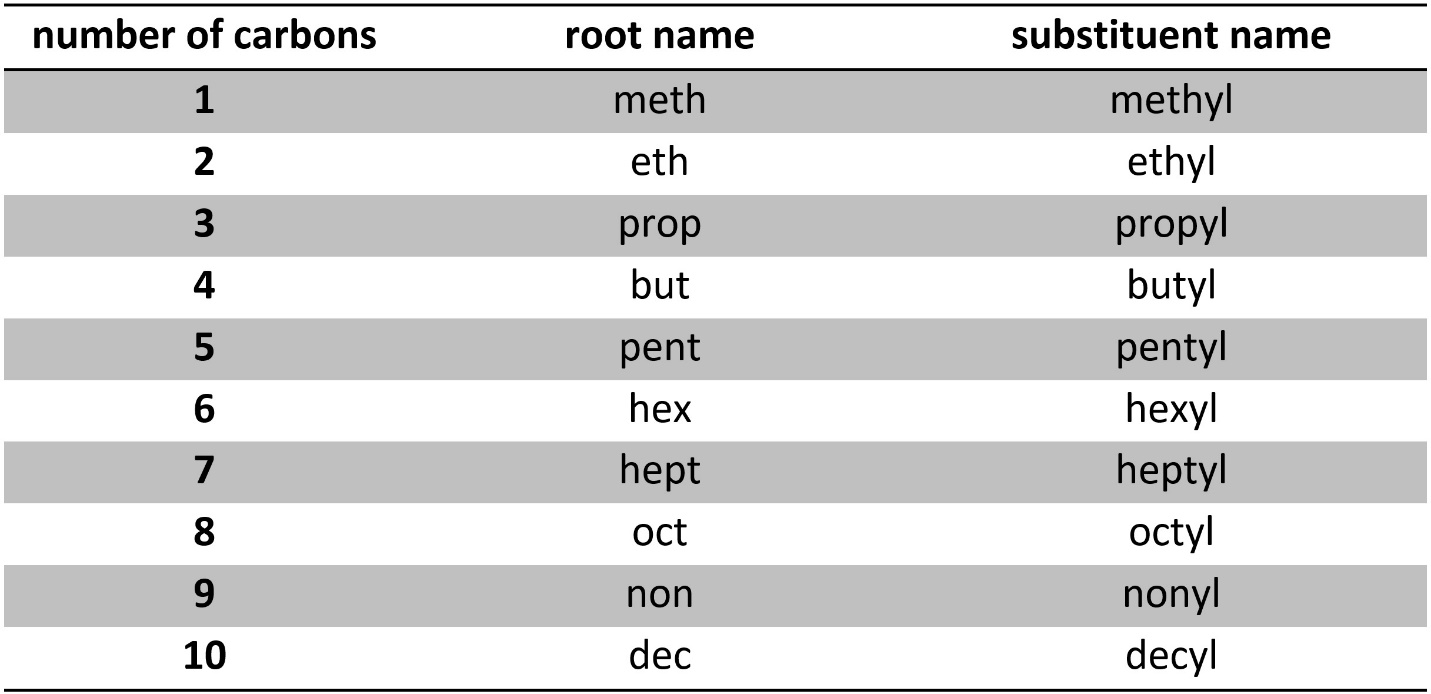
Usually there is more than one place for substituents to be added. As a result, we also add a numerical part to the prefix next to each type of substituent (Figure 2.42). Each carbon in the main chain is numbered in sequence. The numbering is such that the substituents get the smallest number possible.
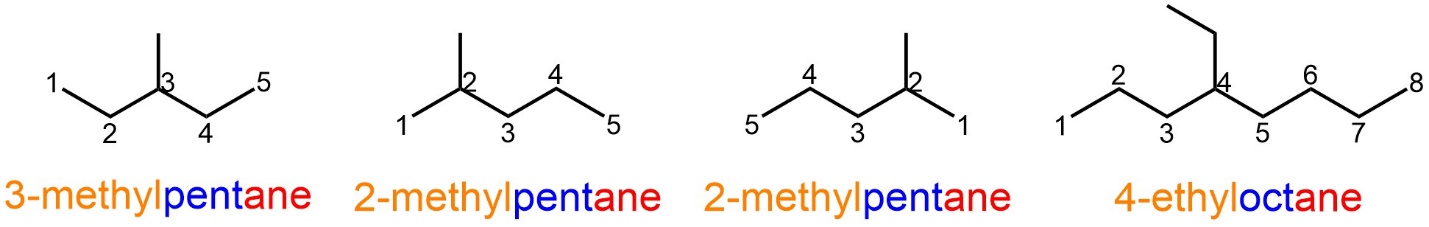
Figure 2.42 – Examples of Named Alkanes with Alkyl Substituents.
Sometimes there are multiple kinds of substituents. The numerical descriptor still goes next to the substituent it describes, and the substituents are listed in alphabetical, NOT numerical, order (Figure 2.43). In some molecules there is ambiguity in how to number the chain: numbering from left-to-right and right-to-left gives the same overall numbers but assigns them to different substituents. There are rules that can be applied in these cases but these add complexity to the system. Molecules like this will not feature in this text.
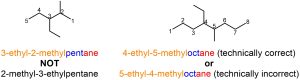
Figure 2.43 – Examples of Named Alkanes with Multiple Different Alkyl Substituents.
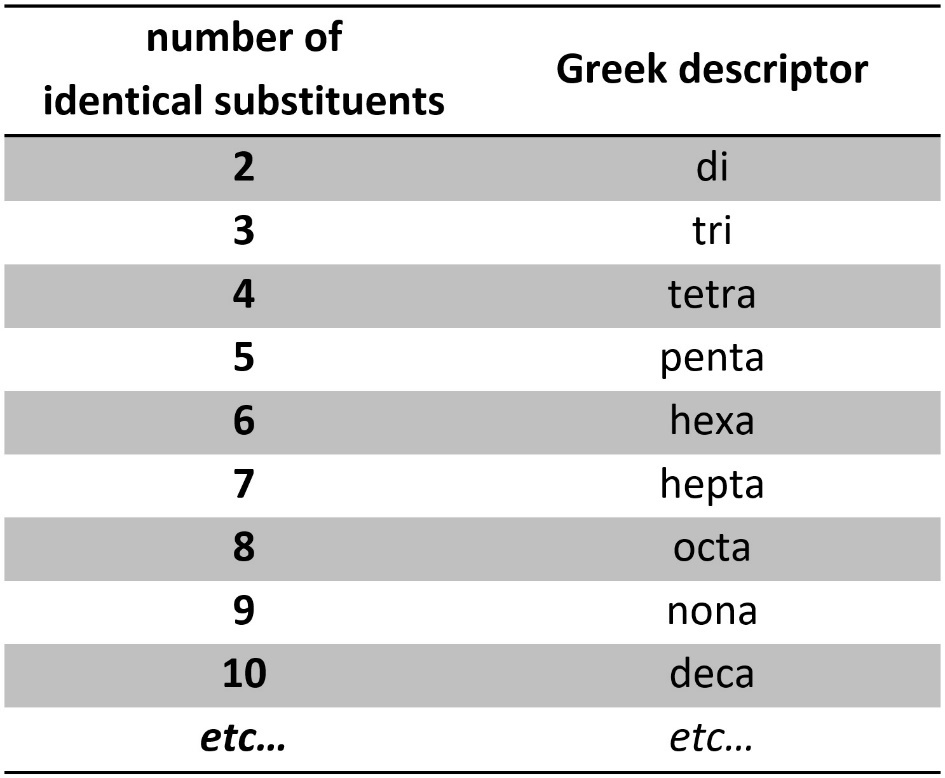
2.5.4. Greek Numerical Prefixes
Sometimes there are multiples of the same kind of substituent. In these cases a Greek descriptor is added to the substituents’ name to indicate how many of the same kind of substituent there are (Table 2.6). As a memorization trick, recall that root names also use Greek words for longer carbon chains; these descriptors are the same but with a vowel added to the end.
Table 2.6 – Greek Descriptors for Multiples of the Same Substituent.
The numerical descriptors still go next to the substituents they describe, but now the Greek numerical descriptor goes between the numbering and the name of the substituent (Figure 2.44).
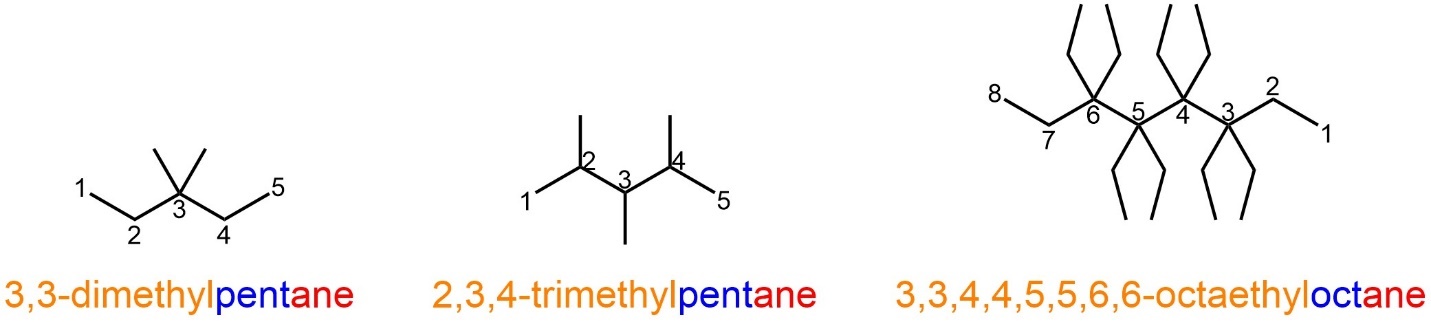
Figure 2.44 – Examples of Named Alkanes with Multiples of the Same Alkyl Substituents.
Confusingly, the Greek descriptors do not count when determining alphabetical order (Figure 2.45). It is also important to remember that more than one kind of substituent is possible at the same position.
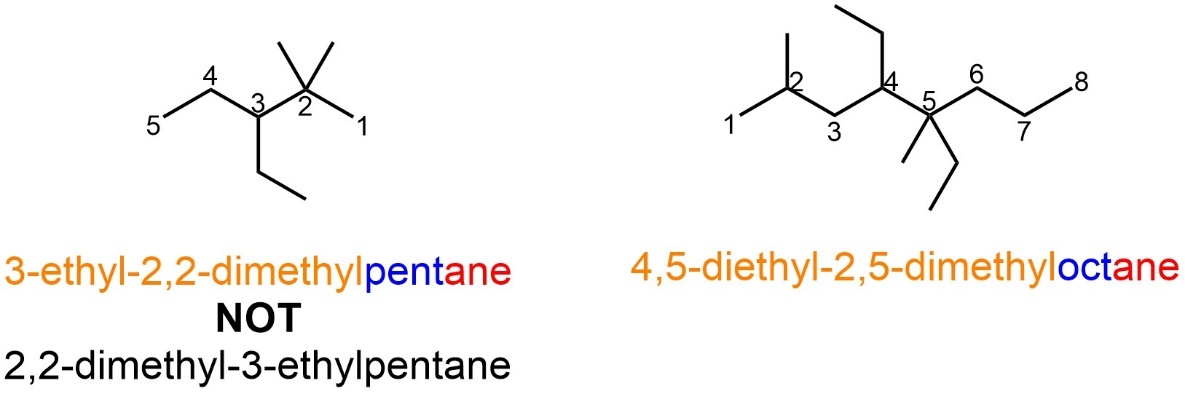
Figure 2.45 – Examples of Named Alkanes with Multiples of Different Alkyl Substituents.
2.5.5. “Advanced” Groups with Trivial Names
As alkanes may have branches (substituents), the substituents themselves may also have branches. There are specific rules for how to name these, but they are not necessary at an introductory level. However, some advanced groups are so common that they have trivial names which are used instead (Figure 2.46). Some of these groups contain other functional groups such as alkenes or aromatic rings, but are included here for convenience.
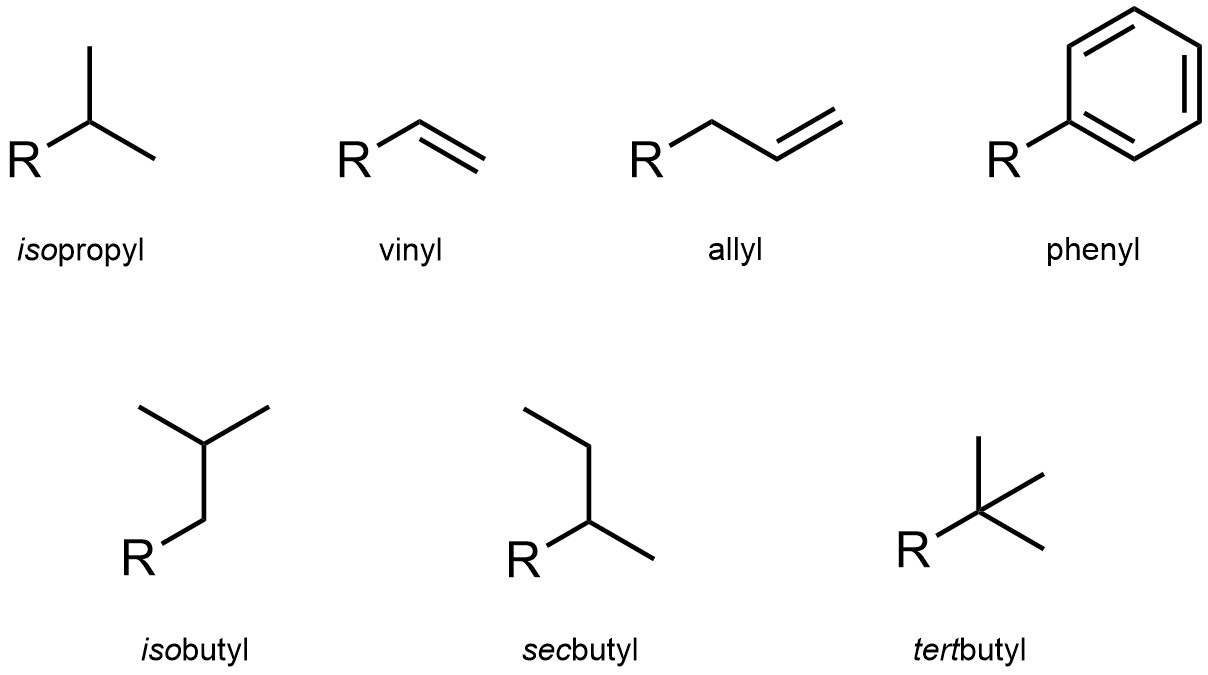
Figure 2.46 – Examples of Trivial-Named Hydrocarbon Substituents.
Trivial names are a form of slang, a specific word that refers to a compound or group that does not fall under the standard systematic rules but is so commonly used that they are recognized as alternatives (see Section 2.5.11.). While the formal rules may be used to name compounds with these groups it is more common (and usually easier) to use the trivial name (Figure 2.47).
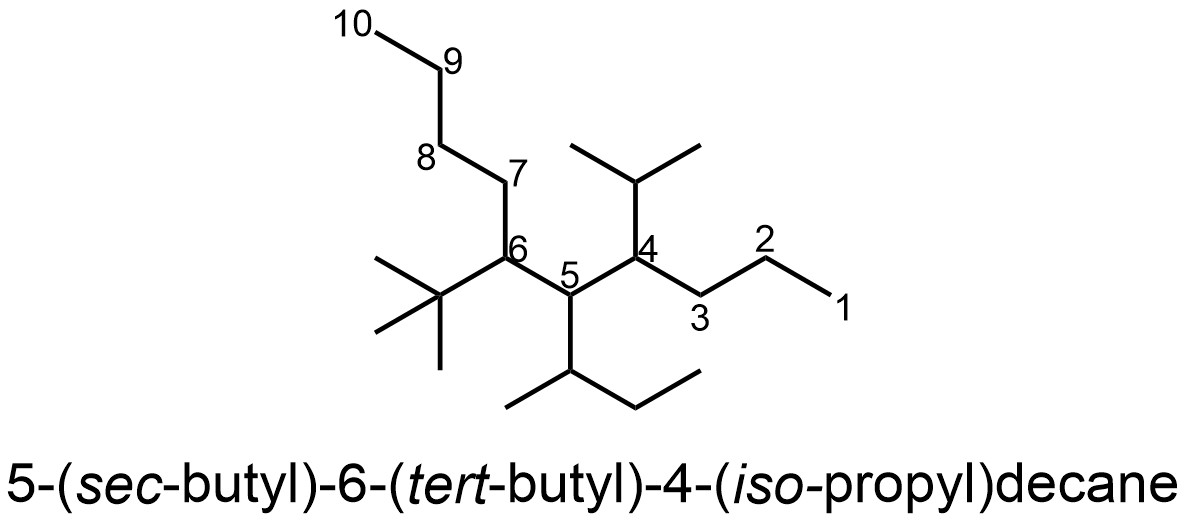
Figure 2.47 – Example of a Named Alkane with Trivial-Named Alkane Substituents.
2.5.6. Cyclic Alkanes
The term “cyclo” is prepended to the root name when the longest contiguous carbon chain is in a ring (Figure 2.48). Substituents can be added using the same rules as before. Be aware that if there is only one substituent it does not get a numerical descriptor for its location (every place on the ring is equivalent).
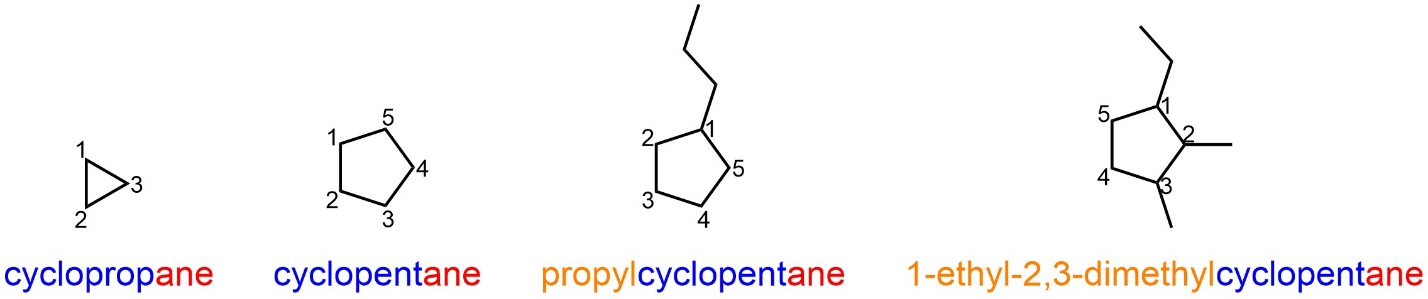
Figure 2.48 – Examples of Named Cyclic Alkanes.
If there is ring substituent on the ring the smaller ring has “yl” appended as before for its substituent name in the prefix (Figure 2.49).
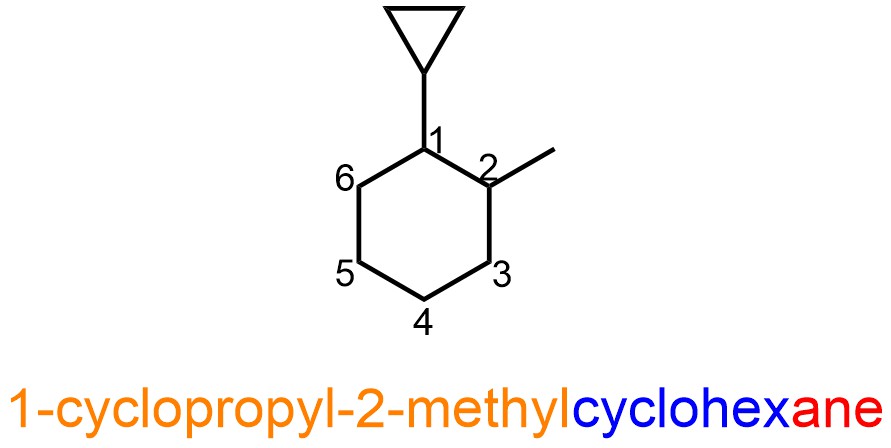
Figure 2.49 – Example of a Named Cyclic Alkane with a Cyclic Alkane Substituent.
There is debate about how compounds should be named if there is a longer contiguous carbon chain in an area outside of the ring system. To avoid ambiguity, molecules like this will not feature in this text (Figure 2.50).
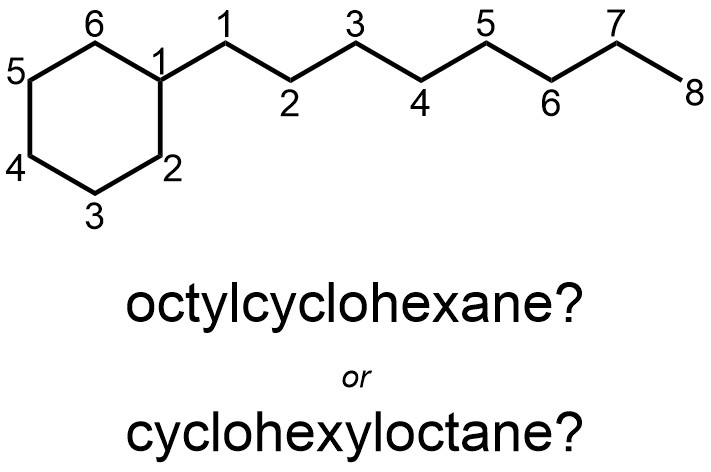
Figure 2.50 – Ambiguous Naming for Cyclic Rings with Extended Alkyl Substituents.
2.5.7. Alkenes and Alkynes
In molecules where alkenes are the only functional group (other than alkanes) they become the principal functional group by default. This means that these compounds will have the suffix “ene”, which represents alkene. Any numerical or Greek descriptors are kept near the “ene” (beside the functional group(s) they are describing) and considered part of the suffix. The numerical descriptor is the number of the carbon where the alkene starts (the lower of the two) and the alkene ends at the next numbered carbon. This also applies to alkenes in rings. All other rules apply as before (Figure 2.51).
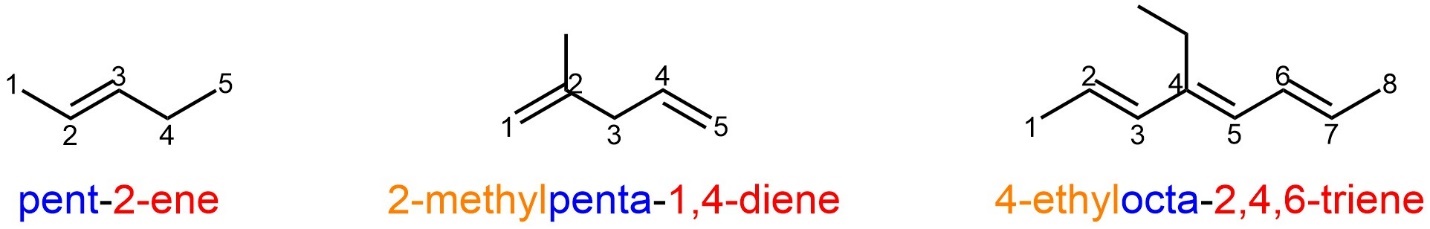
Figure 2.51 – Examples of Named Alkenes with Alkyl Substituents.
In molecules where alkynes are the only functional group (other than alkanes) they become the principal functional group by default. This means that these compounds will have the suffix “yne”, which represents alkyne. Any numerical or Greek descriptors are kept near the “yne” (beside the functional group(s) they are describing) and considered part of the suffix. The numerical descriptor is the number of the carbon where the alkyne starts (the lower of the two). All other rules apply as before (Figure 2.52).
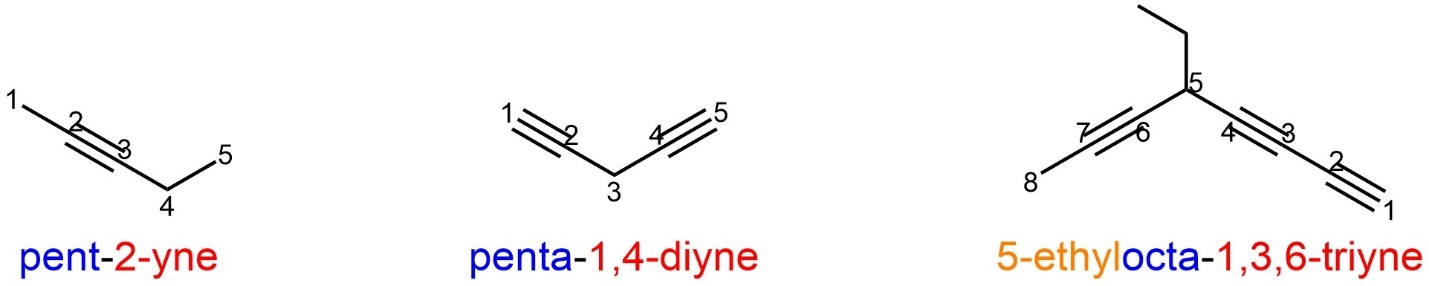
Figure 2.52 – Examples of Named Alkynes with Alkyl Substituents.
In the examples above a set of grammatical rule begins to apply. For instance, in four of the examples above an “a” is added at the end of the root name. While technically correct, it is often difficult at an introductory level to implement grammar. When naming compounds be aware that this is a chemistry text, not an English primer. However, it is important to understand that vowels may be added or removed (see below) for reasons of grammar but that this has no impact on the functional group(s) being described.
Alkenes and alkynes have another special rule for naming: if another functional group has priority over the alkene/alkyne then its description moves to the end of the root name, NOT the prefix. For example, alkynes have priority over alkenes. If a molecule contains both, the suffix “yne” is used and “ene” (and all numerical/Greek descriptors for it) moves to the end of the root name. For reasons of grammar the last ‘e’ is dropped here (Figure 2.53).
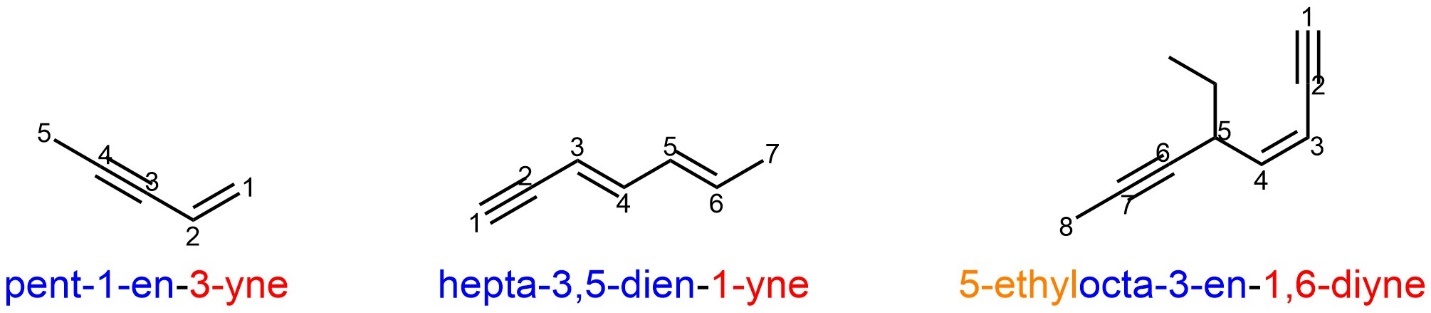
Figure 2.53 – Examples of Named Compounds with Alkenes and Alkynes.
Technically this is also true for alkanes (except when there are also alkenes/alkynes); the “ane” moves to the end of the root name and the last ‘e’ is dropped (see Section 2.5.8. for examples).
2.5.8. Other Functional Groups
There are hundreds of specifically named functional groups. To provide all required details for naming molecules containing just the groups covered in this text (see Section 2.2.) would take several dozen pages. Instead, only a selection of the more common (and more easily named) groups will be covered.
2.5.8.1 Priority and Functional Group Prefixes and Suffixes
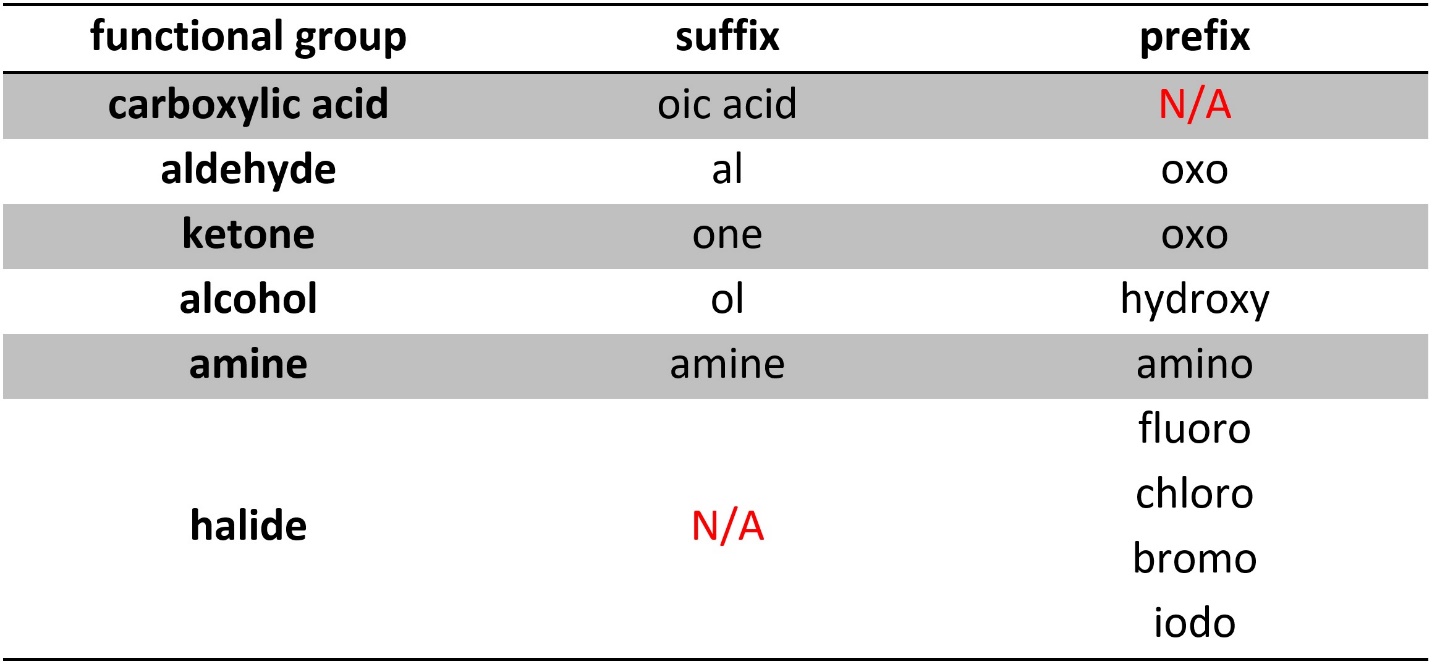
In previous sections ‘priority’ was mentioned several times. All functional groups have prefix and suffix indicators. In a compound’s name the suffix comes from the group with the highest priority. All other functional groups (except alkenes and alkynes, see Section 2.5.7.) in the molecule are described using their corresponding prefix indicators in the prefix of the name. Numbering and other rules apply as usual. As with alkenes/alkynes, numerical and Greek descriptors for the highest priority group(s) go in the suffix.
Priority may be assigned based on the standardized priority ranking set out by IUPAC (Table 2.7). These functional groups are ordered in descending order of priority (highest to lowest).
Table 2.7 – Functional Group Prefixes and Suffixes Ranked in Descending Order of Priority.
Some functional groups, like aldehydes and ketones, have different suffixes but the same prefix. This is uncommon but highlights a potential issue when converting a name into a chemical structure. Halides have no suffix and are considered lower priority than even alkanes for naming. Notice that the introduction of other functional groups can rapidly add a great deal of length to a compound’s name (Figure 2.54). In some cases numbering is omitted, most often for a suffix’s position. There are specific rules for when this applies, but they are not required; adding the number when generating the name is always acceptable. When encountering a name with a missing number assume the group is located at position 1. For example, the carboxylic acid in 3,4-dioxopentanoic acid and the amine in ethanamine are both assumed to be a position 1 in their respective structures.
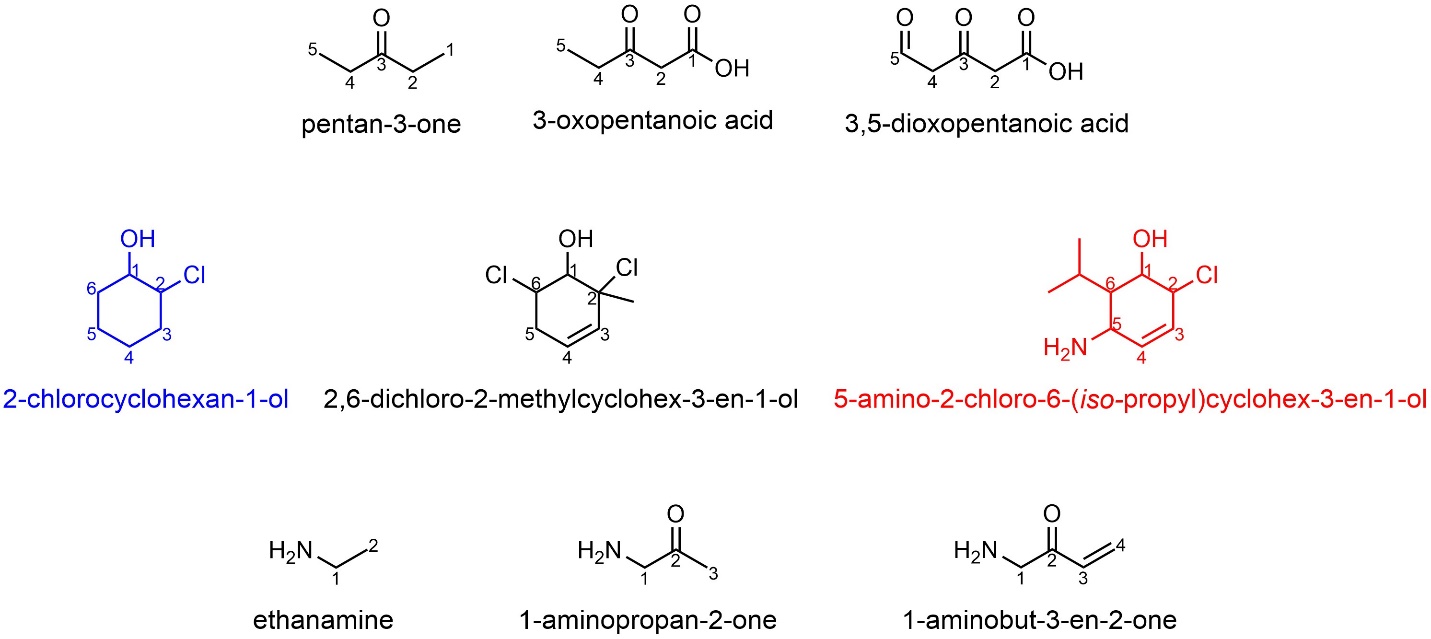
Figure 2.54 – Examples of Named Compounds with Multiple Functional Groups.
2.5.9. How to Determine the IUPAC Names of Simple Compounds
Determining the systematic name for a compound from its structure is straightforward but time consuming. It is much easier to convert a name into a structure (see Section 2.5.10). The first step is always to identify the highest priority functional group(s), then the main chain/ring, and then number the skeleton. After these steps, the easiest approach is to start at the suffix, then determine the root, and only then determine the prefix. If there is stereochemical information (see Chapter 4) it is always best to determine and add this last, once confident in the rest of the name.
Example:
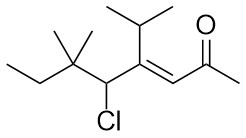
- Identify the highest priority functional group(s).

- Identify and number the main chain/ring.
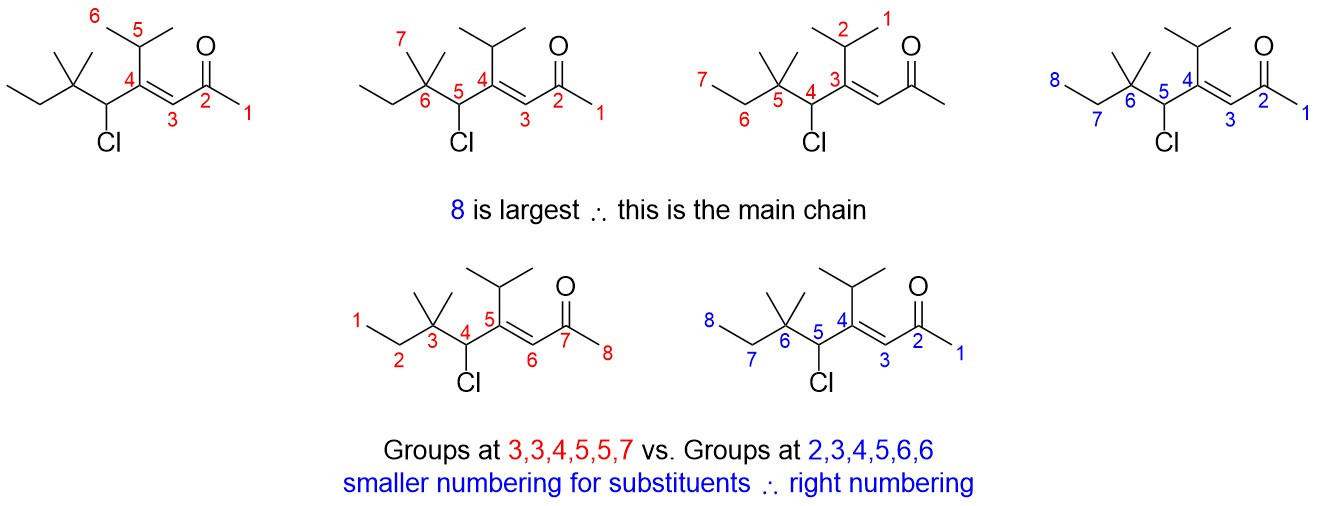
- Determine the suffix.
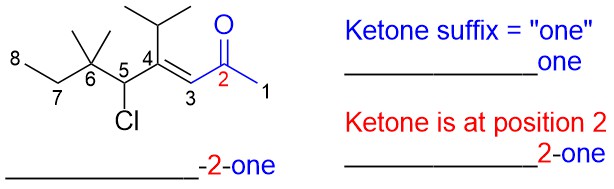
- Determine the root name.
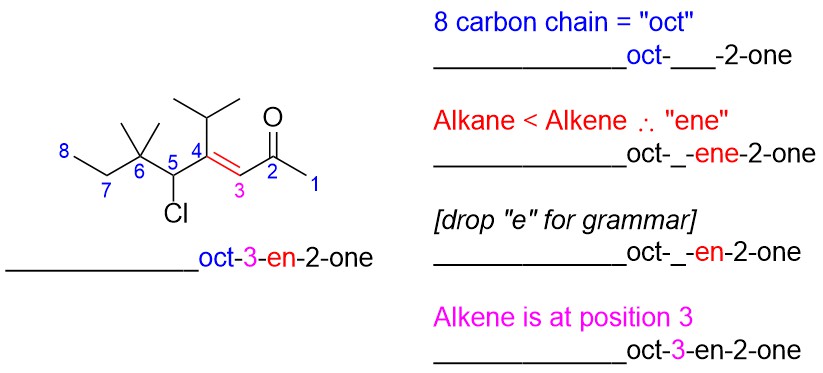
- Determine the prefix. Usually it is easiest to add substituents one by one by going down the chain/ring.

- Add stereochemical information if applicable. See Chapter 4.
Remember that this is meant to allow communication and convey the structure, not be a lesson in pedanticism; forgetting to add some hyphens (or adding extra) and/or failing to add/remove letters for grammar are technically mistakes, but are not usually critical errors. For example, the following would have also been acceptable:
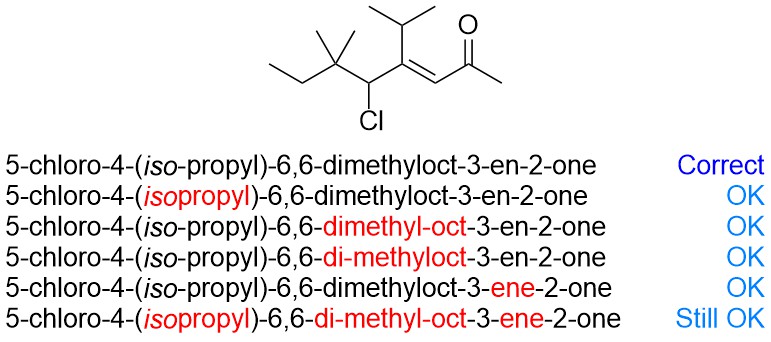
Remember also that the complete rules for naming are excessively long and have many “special cases” and exceptions. At an introductory level there is no need to include ambiguous examples or questions.
2.5.10. How to Draw Chemical Structures from an IUPAC Name
Unlike the converse, determining a structure from a name is relatively easy. Usually the best approach is to first use the root name to draw out the ‘skeleton’, numbering carbons down the chain/ring. Then add the highest priority group from the suffix, and finally add in substituents using the prefix. Remember that the name inherently contains all information needed, but that some critical thinking might be needed. For example, whether an “oxo” is going to be an aldehyde, ketone, or both depends on how many there are and where the are positioned on the skeleton. As before, if there is stereochemical information (see Chapter 4) it is always best to determine and add this last, once confident in the rest of the structure.
Example: 3-butyl-1,2,6-trimethylcyclohex-4-ene-1,2-diamine
- Use the root name to draw out the ‘skeleton’. Since there are no substituents yet, number the carbons arbitrarily (i.e. either end in a chain can be 1, any position in a ring can be 1).
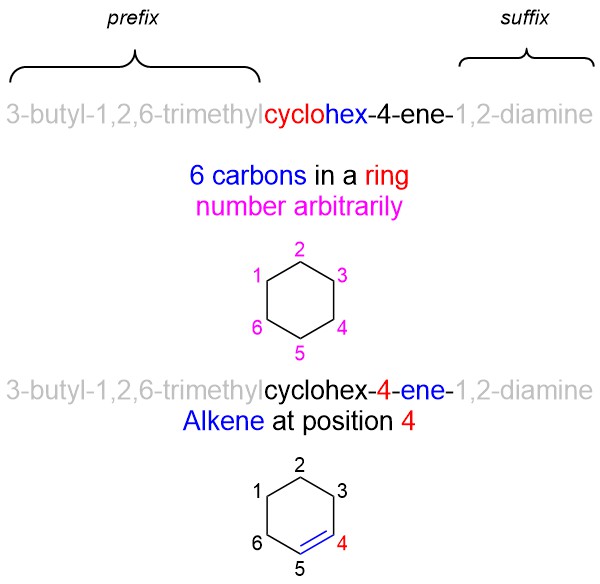
- Use the suffix to add the highest priority group(s)
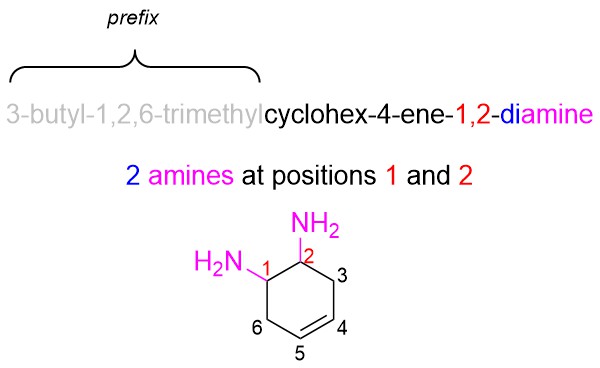
- Add substituents using the prefix. The order substituents are added to the structure does not matter. Adding them in a systematic way helps ensure none are missed.
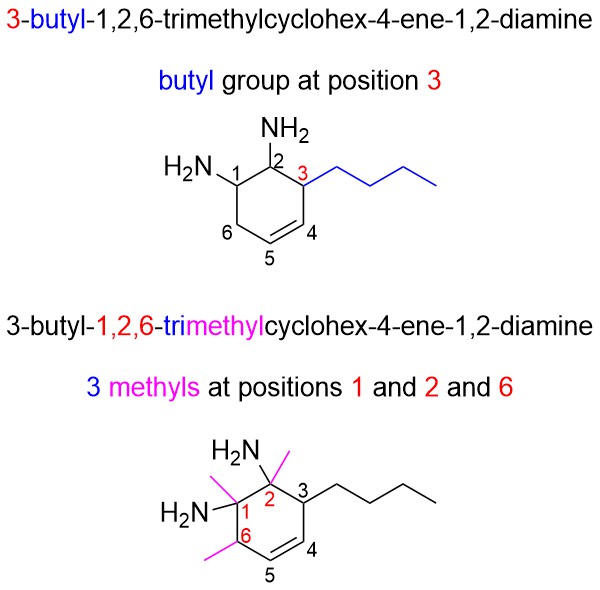
- Add stereochemical information if applicable. See Chapter 4.
Since every part of the name is used constructing the structure it may be beneficial to underline or highlight portions of the name as they are dealt with. This ensures all parts are added and no components are analyzed more than once.
2.5.11. Abbreviations, Limitations of the IUPAC System, and Trivial Names
Re-drawing the same groups in many diagrams becomes tedious. Occasionally chemists find it convenient to abbreviate groups using short letter codes (Table 2.8). Sometimes more than one way of abbreviating the group is common.
Table 2.8 – Common Abbreviations for Functional Groups.
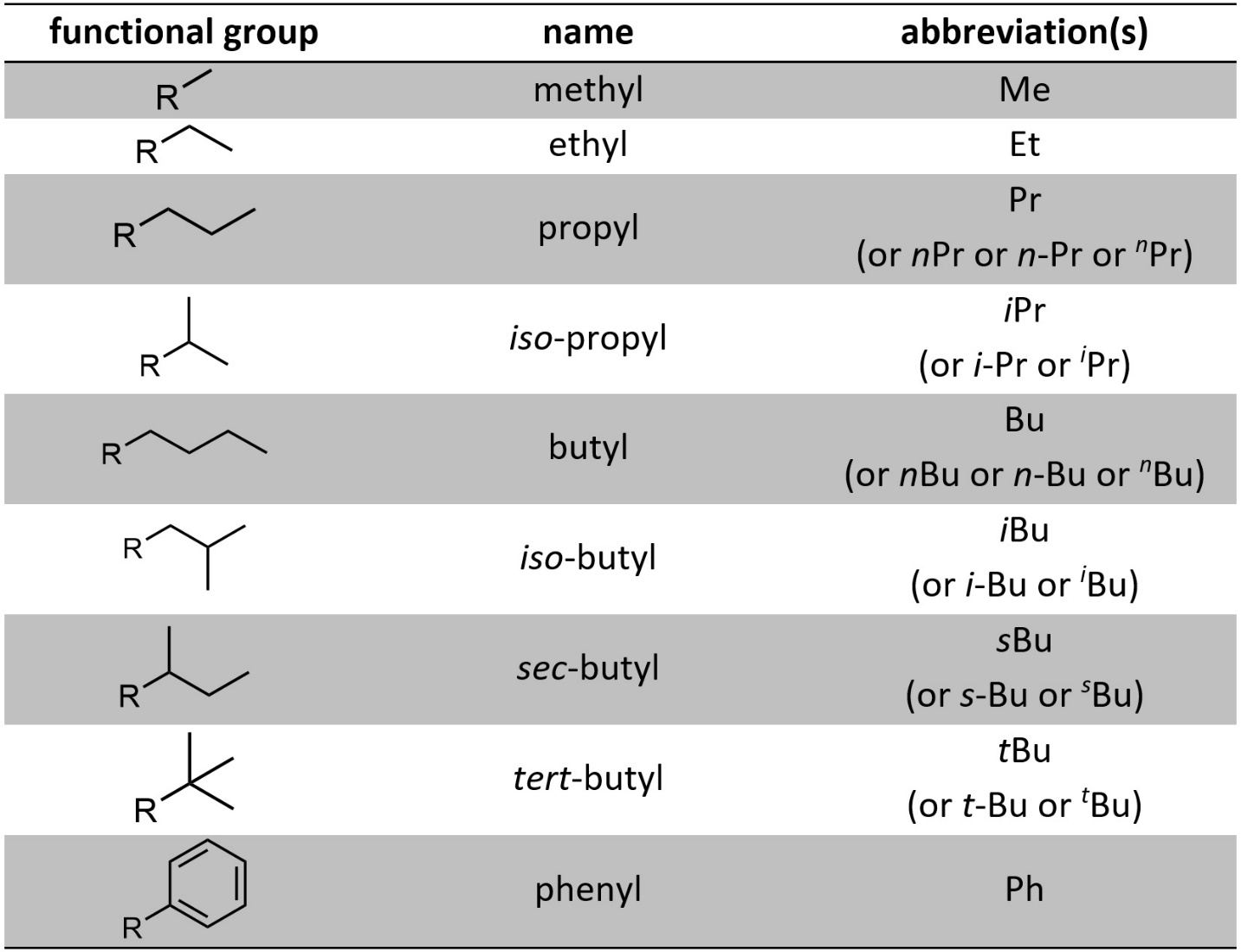
While it is not necessary to use these abbreviations they should be readily understood when encountered (Figure 2.55)
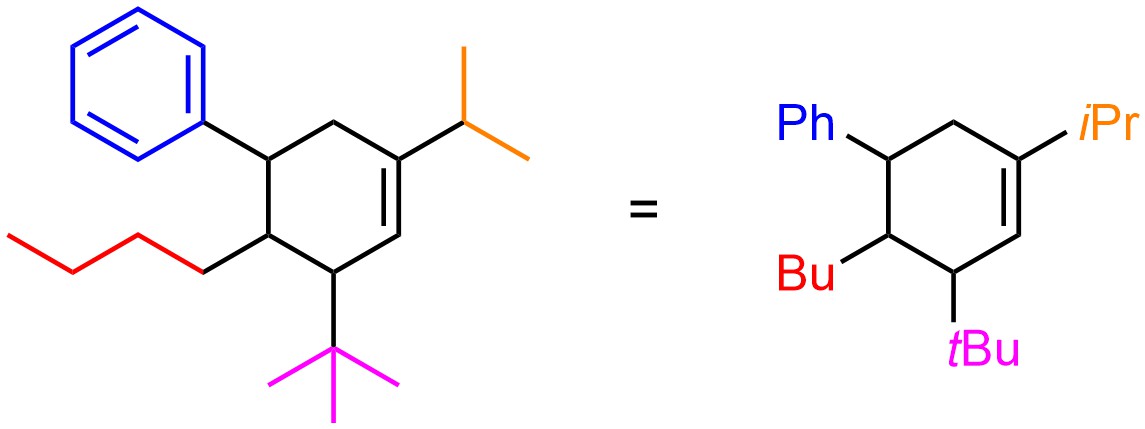
Figure 2.55 – Example of a Compound Drawn with Abbreviated Functional Groups.
There are three situations where chemists actively avoid using the IUPAC nomenclature system.
If communication involves images often compounds are labelled and the IUPAC system is avoided because using the full name does not aide understanding (Figure 2.56). These labels are generally kept within the media being discussed and are not carried between media. For example, the label “Compound A” will (probably) refer to different compounds in different research papers.
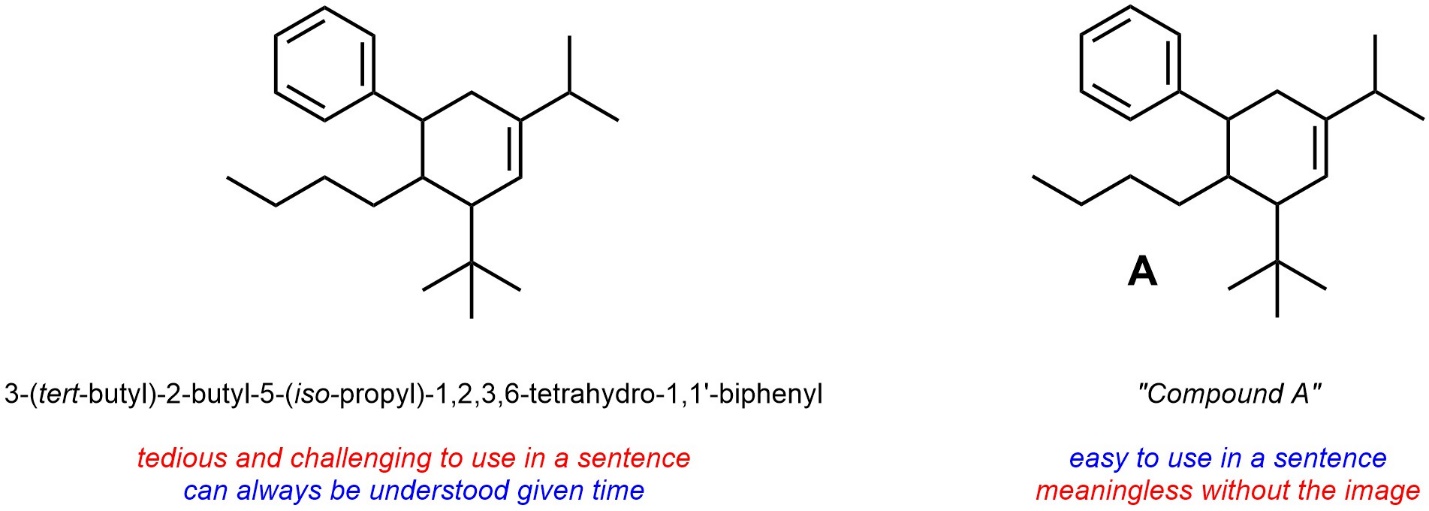
Figure 2.56 – Using Compound Labels in Place of IUPAC Nomenclature.
The other two situations involve the use of trivial names. Recall that trivial names are a form of slang, a specific word that refers to a compound or group that does not fall under the standard systematic rules but is so commonly used that they are recognized as alternatives.
Trivial names for compounds are typically used when the compound is ‘common’, especially with bioorganic molecules (Figure 2.57). For example, most chemists would prefer to use the name “estrogen” to the full IUPAC name for the compound. This may be considered a limitation of the IUPAC system: for many molecules the full name is not practical to use in conversation.
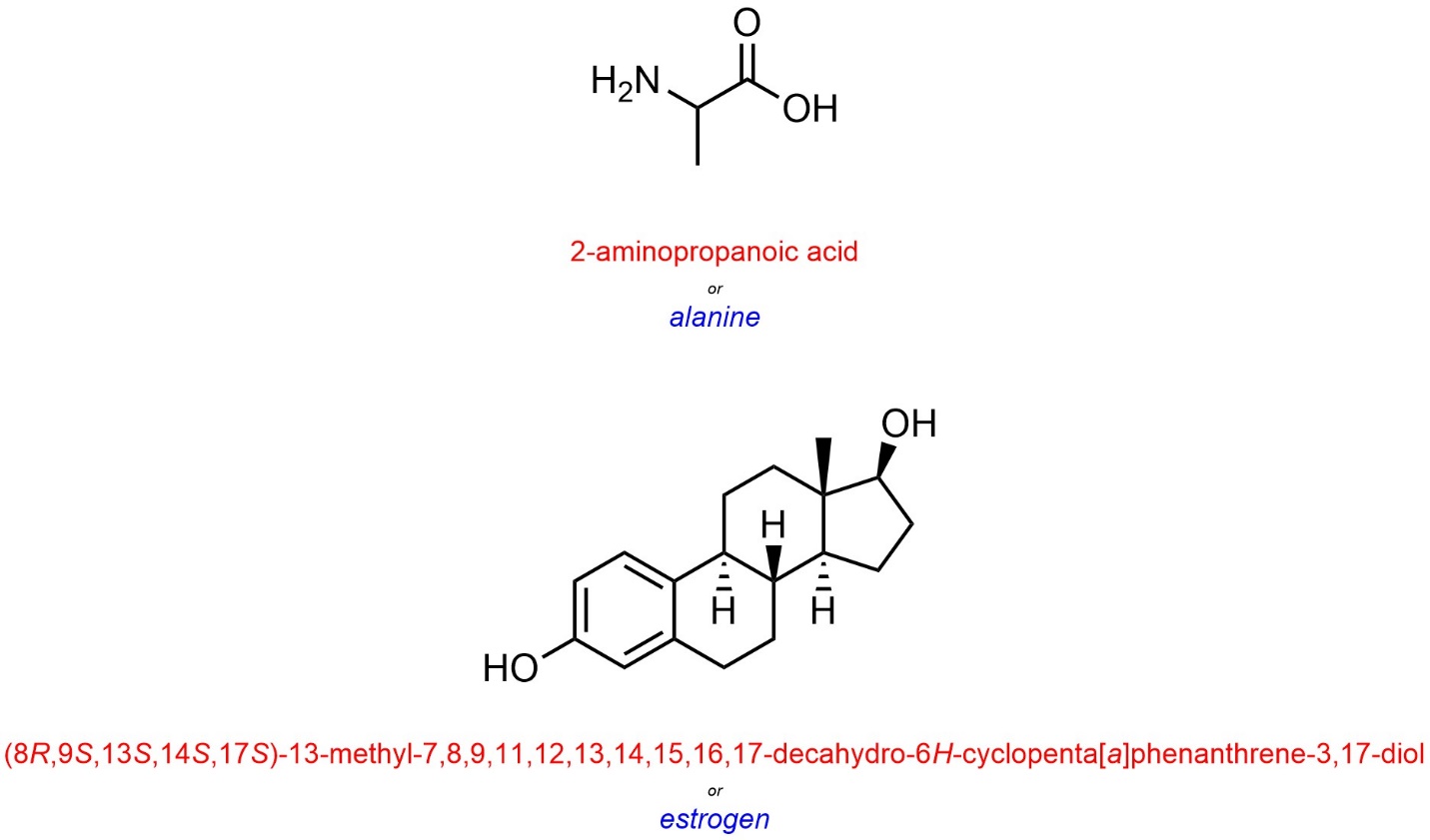
Figure 2.57 – Examples of Using Trivial Names for Common Biomolecules.
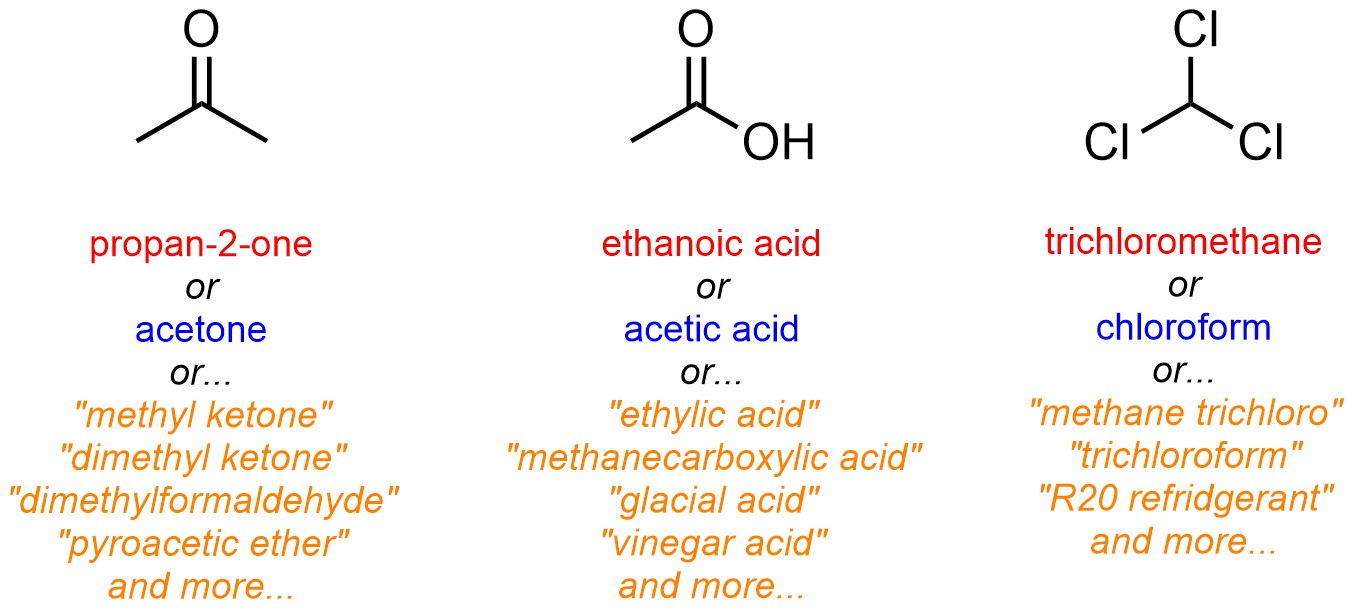
Trivial names are also common for small molecules that have historical significance. Many trivial names were generated for compounds before the IUPAC system was developed. Often the use of these names continues for ‘tradition’ more than convenience (Figure 2.58).
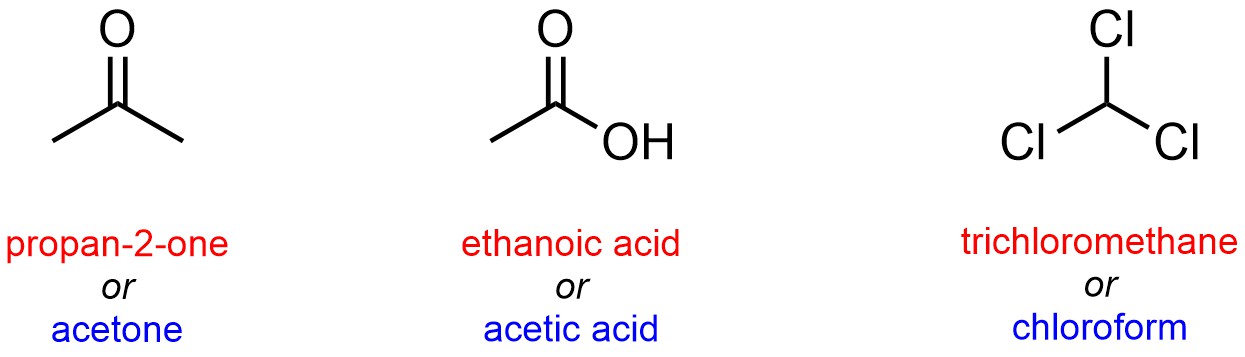
Figure 2.58 – Examples of Using Trivial Names for Common Small Molecules.
In addition to trivial names many molecules have other ‘slang’ names that are semi-commonly used (Figure 2.59). These names will be avoided in this text, but it is important to recognize that sometimes individuals may use formal-sounding names that are not actually a part of the IUPAC nomenclature system.