Chapter 11 Practice Problems – Answers
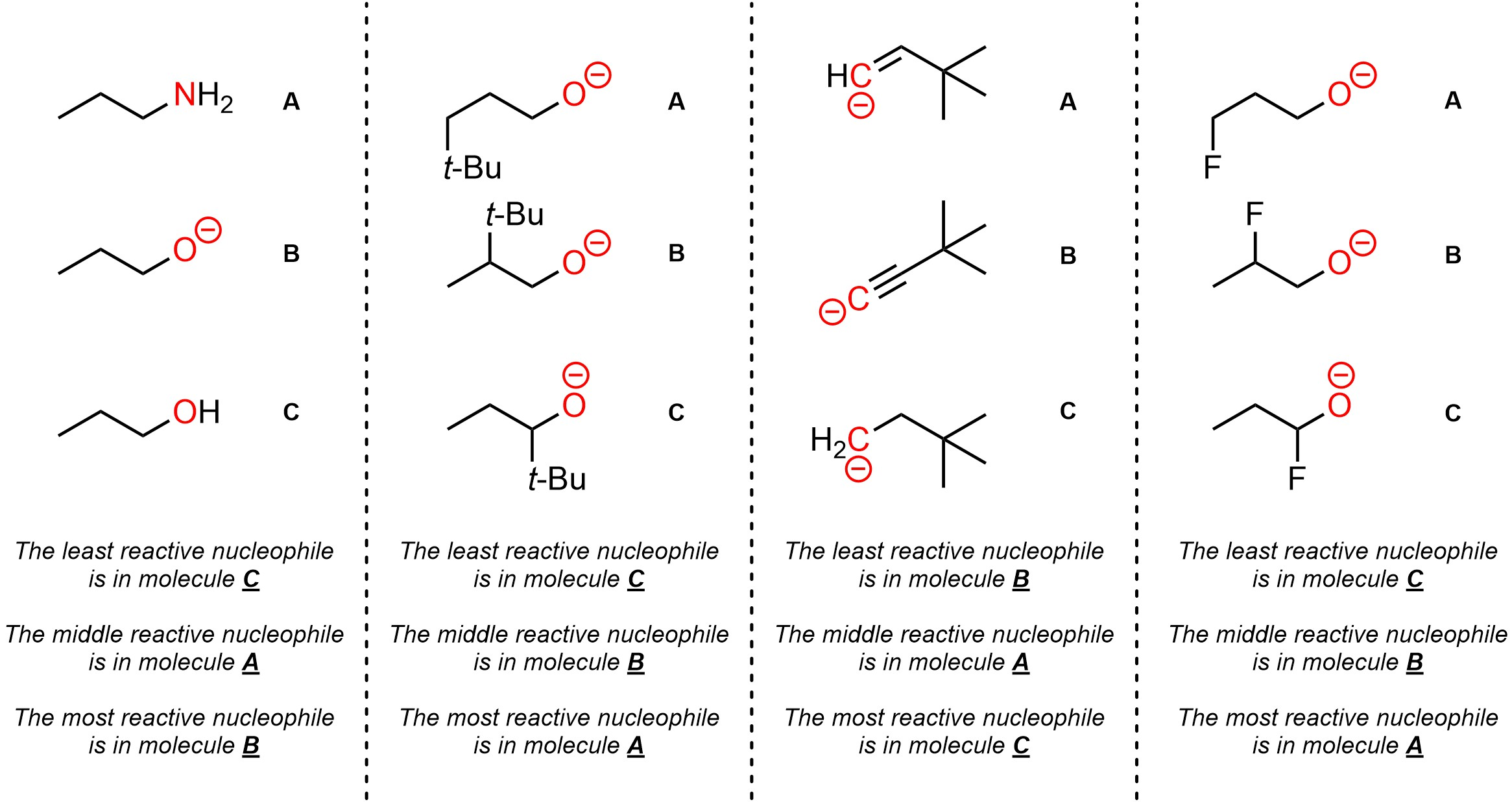
Q11.1: Rank each set of nucleophiles in increasing order of nucleophilicity (weakest to strongest). The nucleophilic atoms to consider are highlighted.
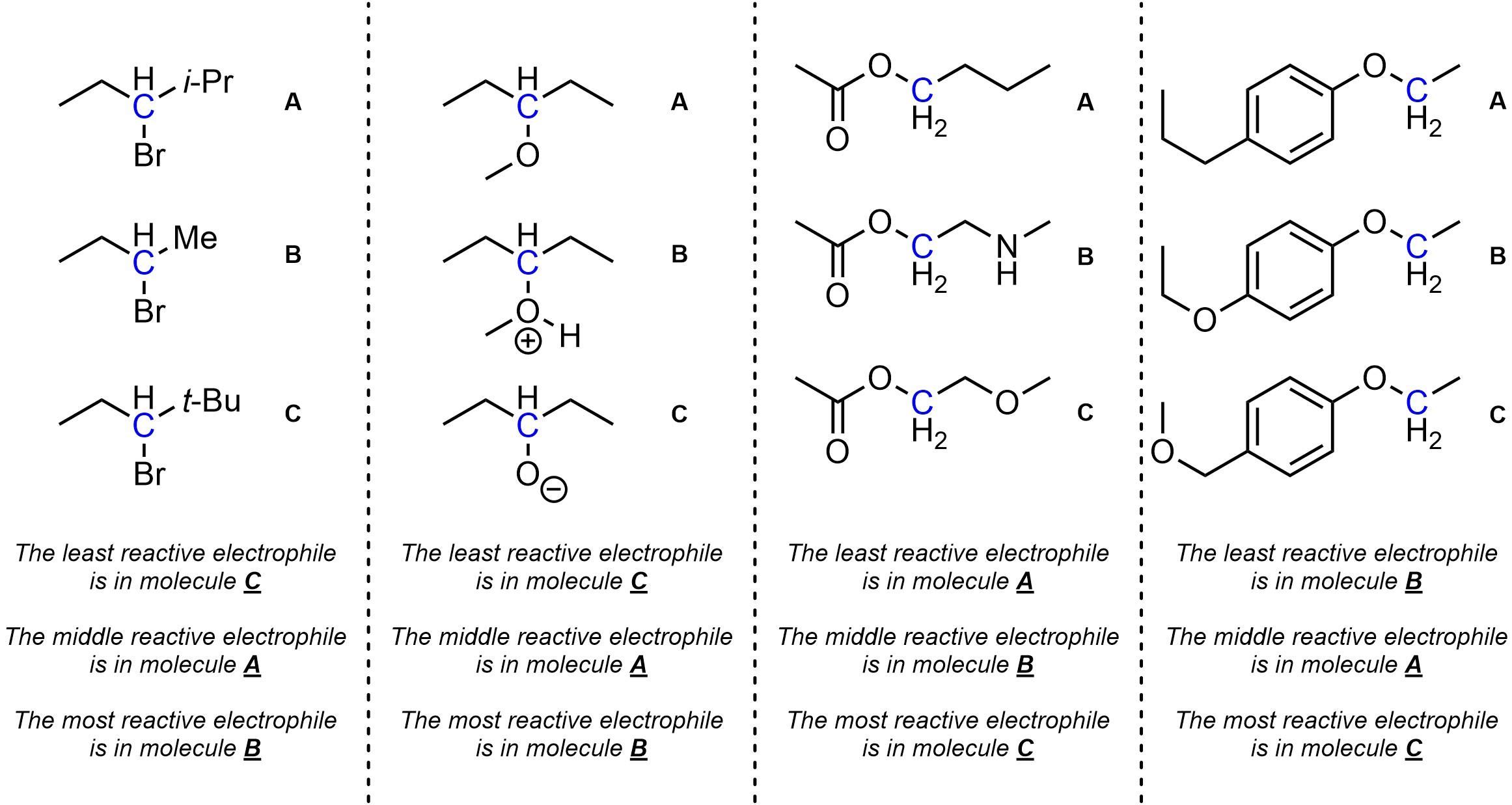
Q11.2: Rank each set of electrophiles in increasing order of electrophilicity (weakest to strongest). The electrophilic atoms to consider are highlighted.
The fourth problem is challenging.
Classing and comparing the para substituents on the aromatic rings
(EDG/EWG) will help.
Thinking about resonance in the intermediate may also help.
Think critically about the effect this has on acidity
(and, by extension, leaving group ability)
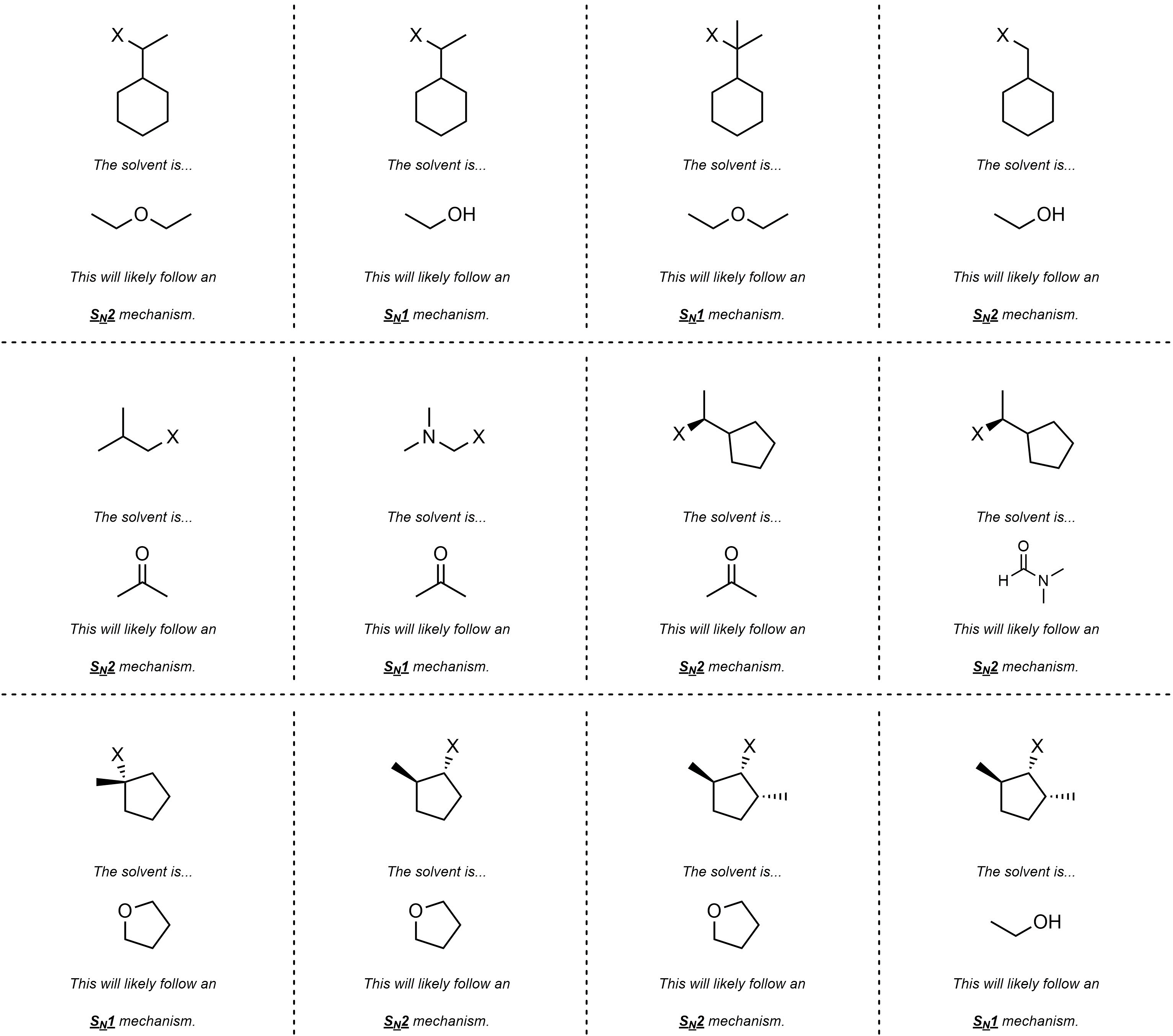
Q11.3: Below are sets of starting materials (electrophiles) and solvents for hypothetical nucleophilic substitution reactions. Determine whether each reaction would likely follow an SN1 or SN2 mechanism.
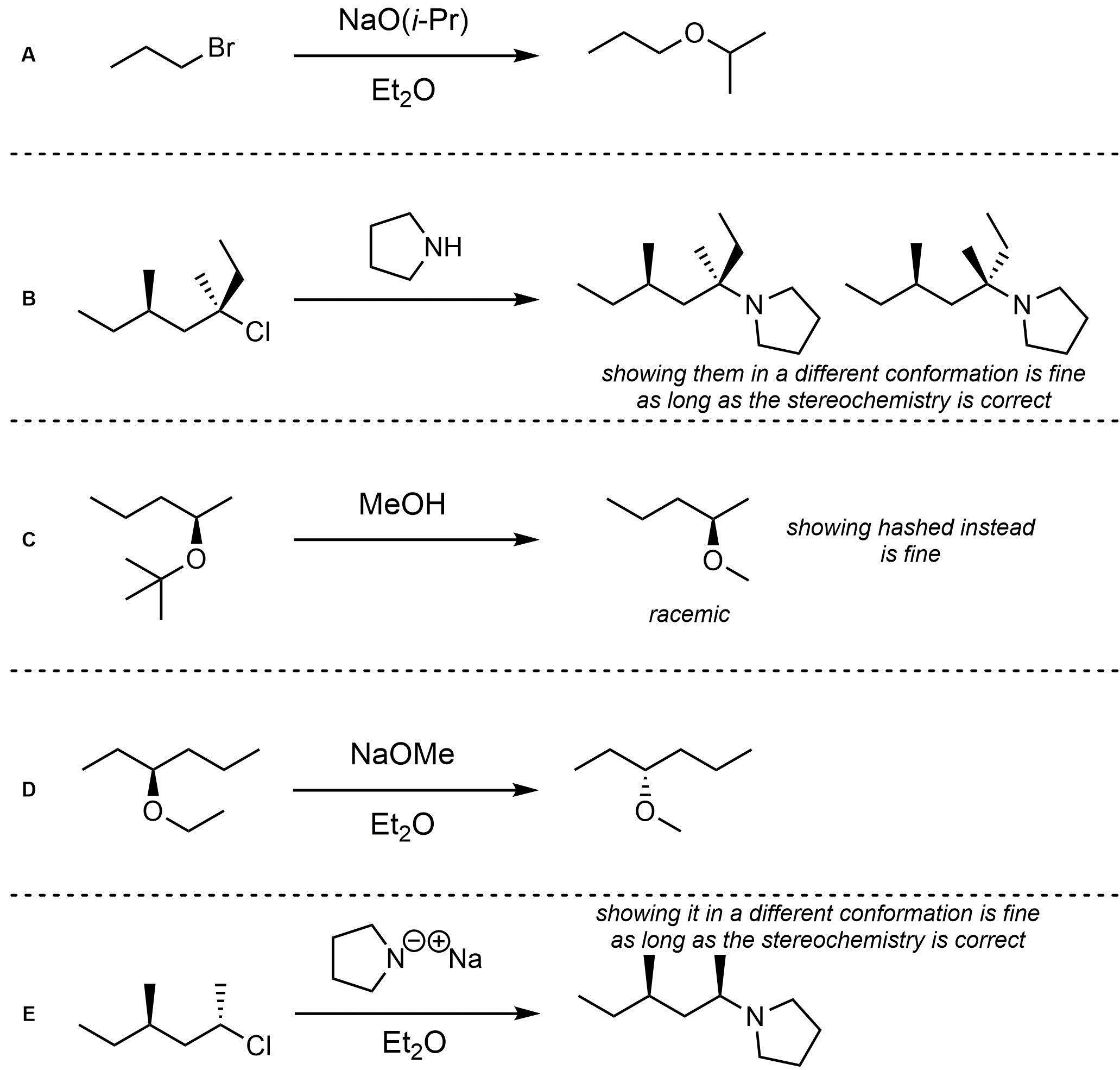
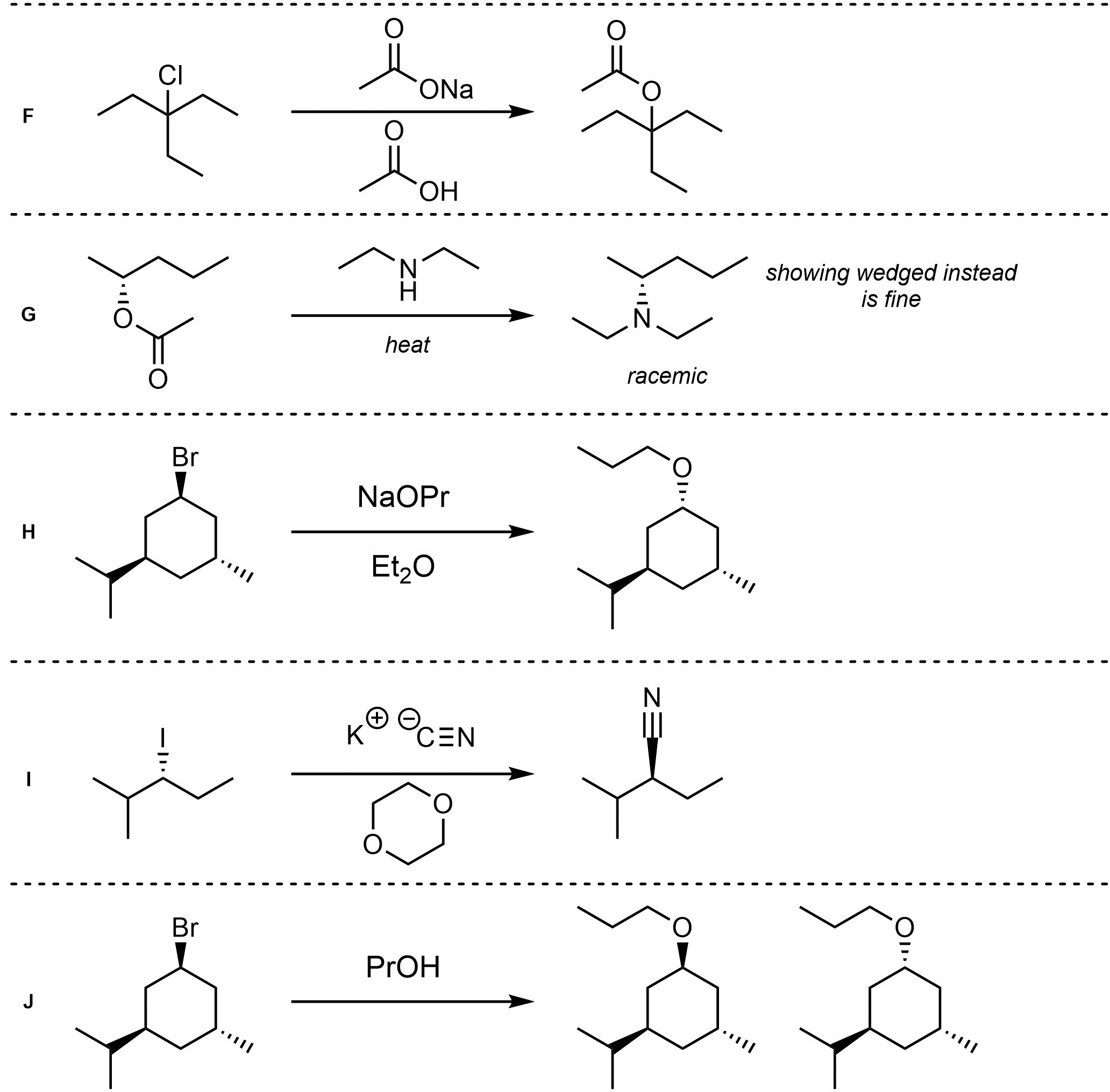
Q11.4: Assume each of the following is a nucleophilic substitution reaction. For each reaction draw the expected major organic product(s). The products must be drawn as line-angle structures. Clearly indicate the appropriate stereochemistry using hashed and wedged bonds where necessary. If the product(s) is/are formed as more than one diastereomer, draw all diastereomers formed. If the product(s) is/are formed as a racemate (racemic mixture) draw only one enantiomer and write the word “racemic” beneath/beside it. Do not write “racemic” if it does not apply.
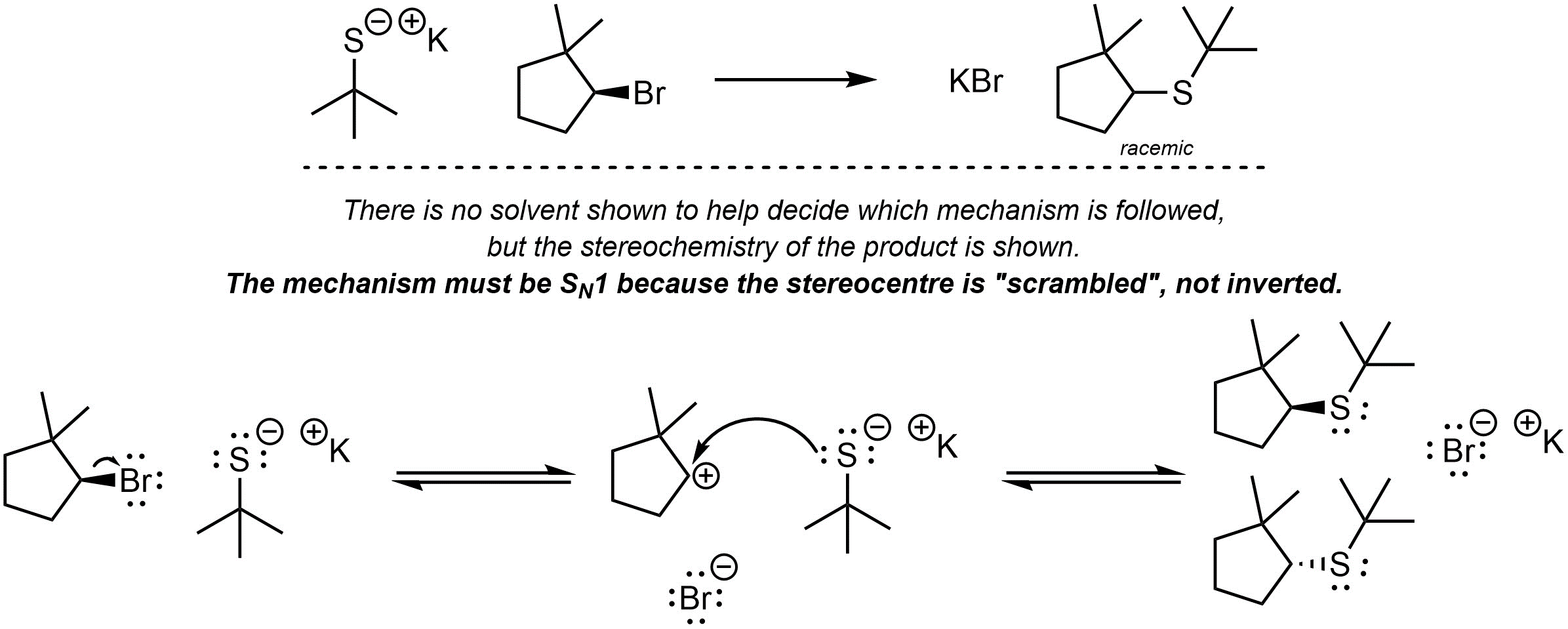
Q11.5: A reaction equation is given below. Write a brief sentence explaining which nucleophilic substitution mechanism is being followed and how you determined this. Then propose a reasonable mechanism for the reaction. Show all necessary intermediates, curved arrows, lone pairs, and formal charges.
Must show and explain SN1 mechanism. SN2 is incorrect.
There are other ways of
indicating the product is racemic when drawing the mechanism.
Using any approach is fine as long as it does not imply it is NOT racemic.