12.3. Elimination Reactions: E1 Reactions
Elimination reactions can occur with one or two steps in the mechanism. Reactions that follow the E1 mechanism have two steps. The main difference from their counterpart is that they do not have a geometric requirement to occur.
12.3.1. Mechanism
Elimination reactions that follow the E1 mechanism have two steps (Scheme 12.8). The leaving group leaves, generating a carbocation. This greatly increases its acidity. Then, the base removes a proton, creating a new conjugate acid and forming a new π bond to the adjacent carbon. The old bond to the leaving group breaks before the new π bond forms.
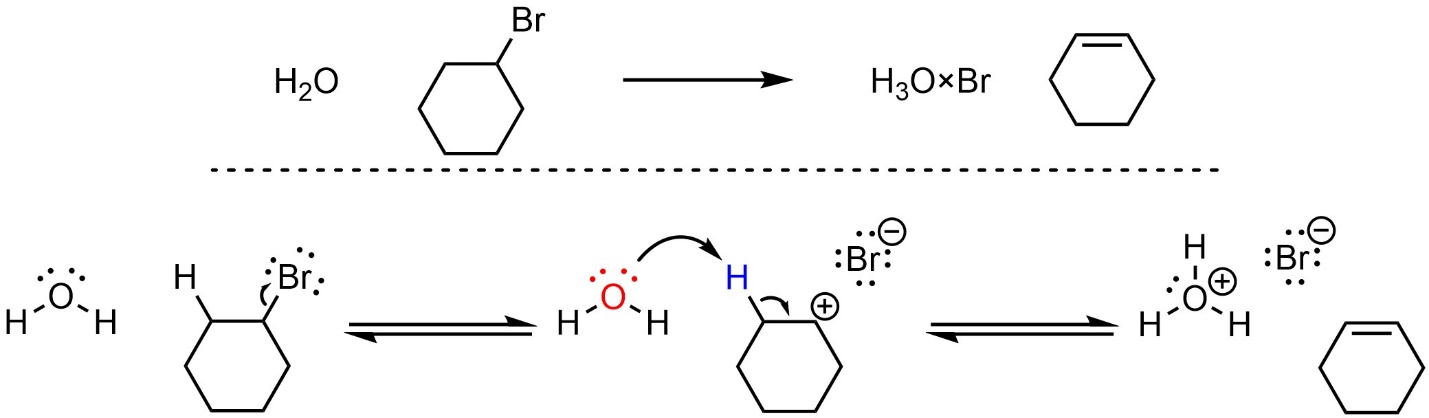
Scheme 12.8 – Reaction Mechanism for E1 Elimination of Bromocyclohexane with Water.
Depending on the specific reaction that is occurring there may be additional steps before or after the substitution step. For example, the nucleophile/base or leaving group may be activated prior to the substitution, or there may be a protonation or deprotonation after the elimination. These extra steps do not change the classification of the mechanism for the elimination step; if the new π bond forms after the old bond to the leaving group breaks, it is still classed as an E1 elimination reaction.
12.3.2. Reaction Coordinate and Rate-Determining Step
The first step, carbocation formation, requires a large amount of energy and is the rate-determining step (Figure 12.4). The overall reaction is slightly exergonic.
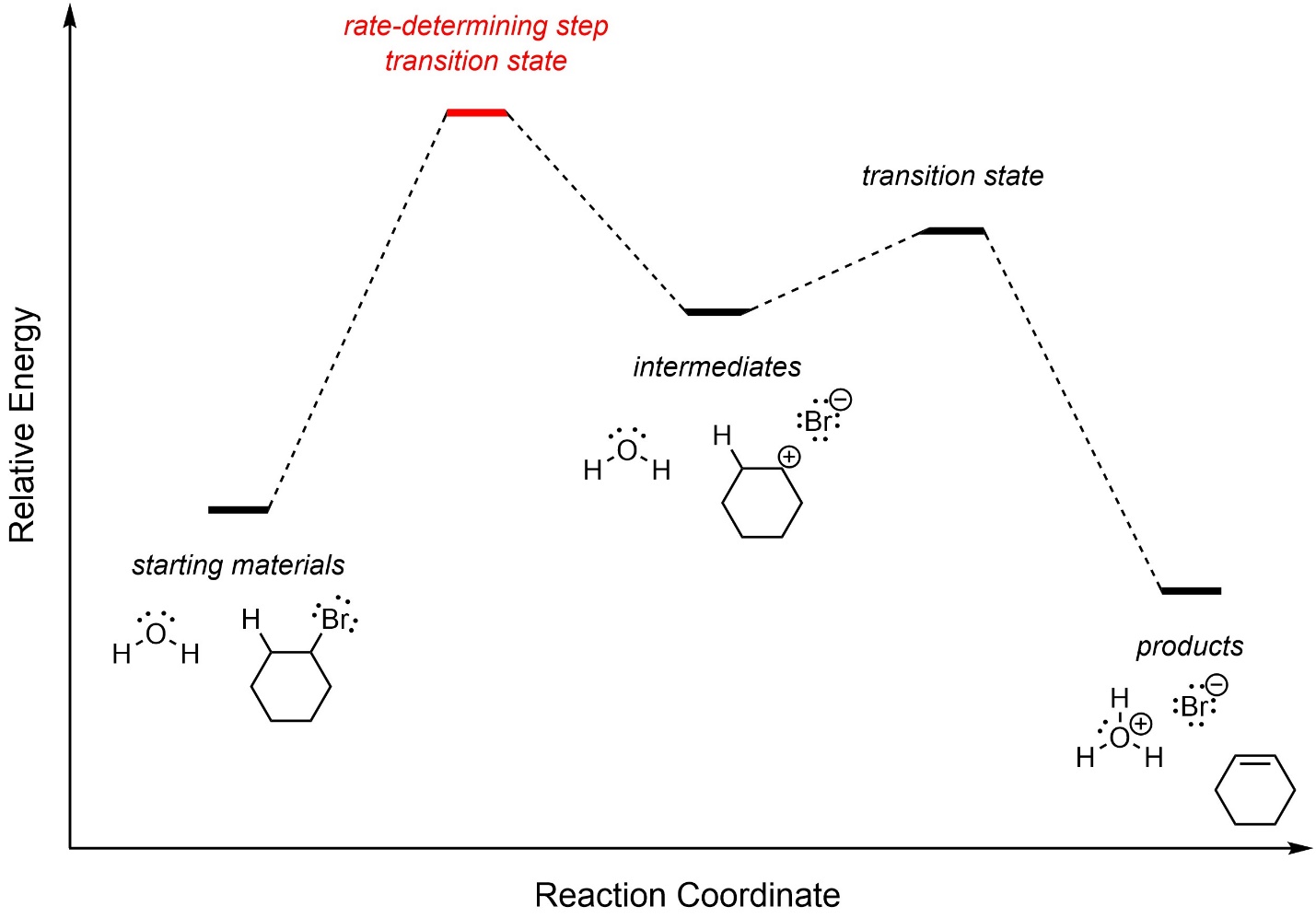
Figure 12.4 – Reaction Coordinate for E1 Elimination of Bromocyclohexane with Water.
12.3.3. Regioselectivity – Zaitsev’s Rule
As with E2 eliminations, E1 eliminations follow Zaitsev’s Rule: the major regioisomer formed will be the most thermodynamically stable one. Generally, the more substituents an alkene has the more thermodynamically stable it is. Gaining additional stabilization through delocalization/resonance still applies (see Section 12.2.4.1). Theoretically, regioselectivity in E1 elimination reactions can be altered by use of a very bulky base (see Section 12.2.4.2). In practice this works exceptionally poorly in almost all cases; this text will not feature examples of these types of reactions.
A major difference between E2 eliminations and E1 eliminations is in the regioselectivity of eliminations from six-membered rings. Reactions that follow E2 mechanisms can have altered regioselectivity as a result of the geometric constraint placed on them (see Section 12.2.4.3). Because the first step in E1 mechanisms is the formation of a trigonal planar carbocation there is no geometric constraint placed on which hydrogen(s) can be removed (Scheme 12.9). As a result, E1 eliminations from six-membered rings can always selectively form the more stable alkene.
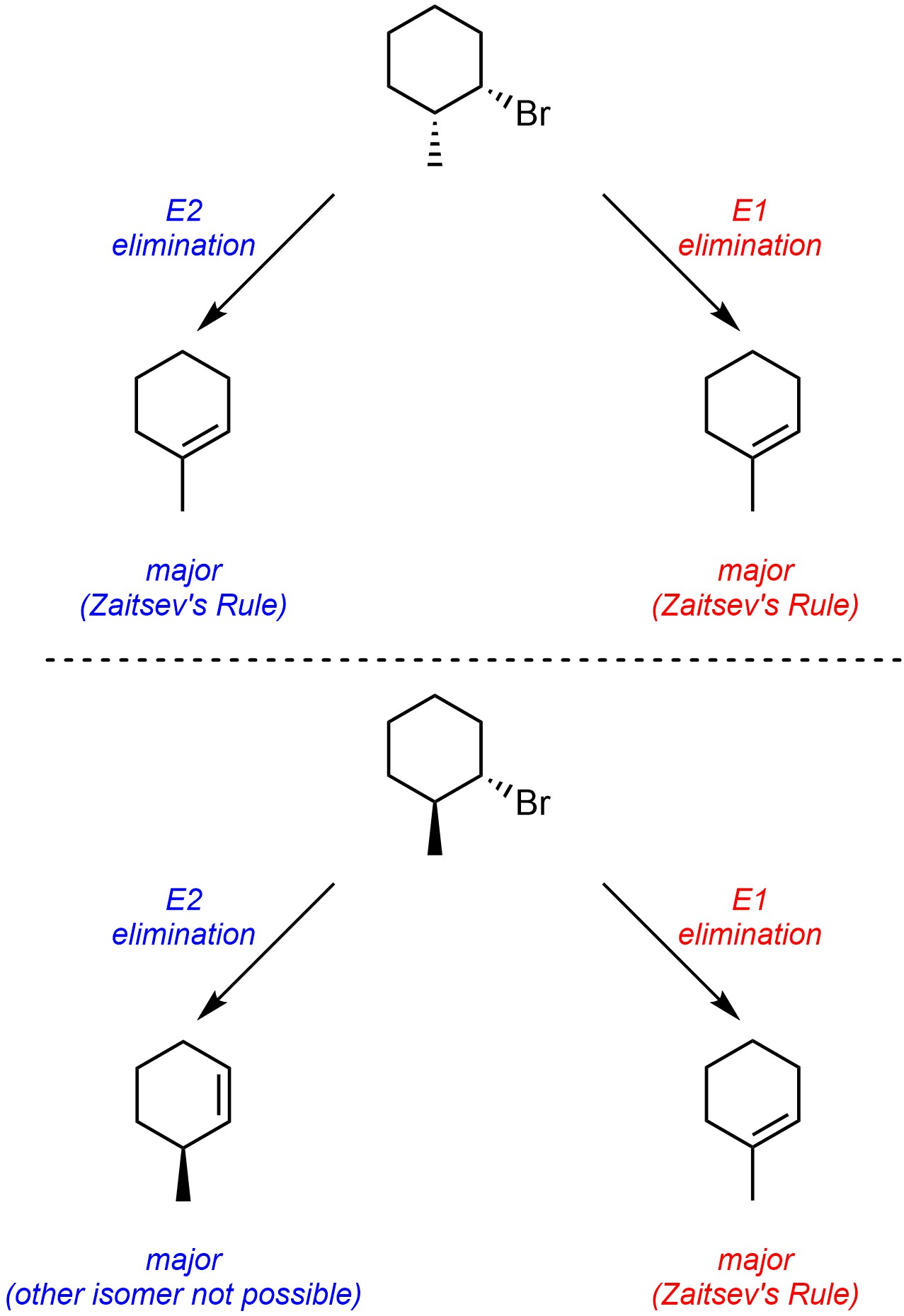
Scheme 12.9 – Contrasting Regioselectivity in Elimination Reactions from Six-Membered Rings Through E2 and E1 Mechanisms.
12.3.4. Stereoselectivity
Reactions that follow the E1 elimination mechanism are stereoselective for the formation of alkenes where the largest groups on either end of the alkene are trans relative to each other. Usually this means they selectively form E-alkenes. However, these reactions are typically less stereoselective than E2 eliminations; depending on the exact structure of the starting material, the selectivity can be very close to 1:1 E:Z. The reasons for this are a competition between kinetic and thermodynamic factors, which lies outside the scope of this text. Unless otherwise indicated, assume reactions that follow E1 mechanisms have the same stereoselectivity as those that follow E2 mechanisms.

