Chapter 12 Practice Problems – Answers
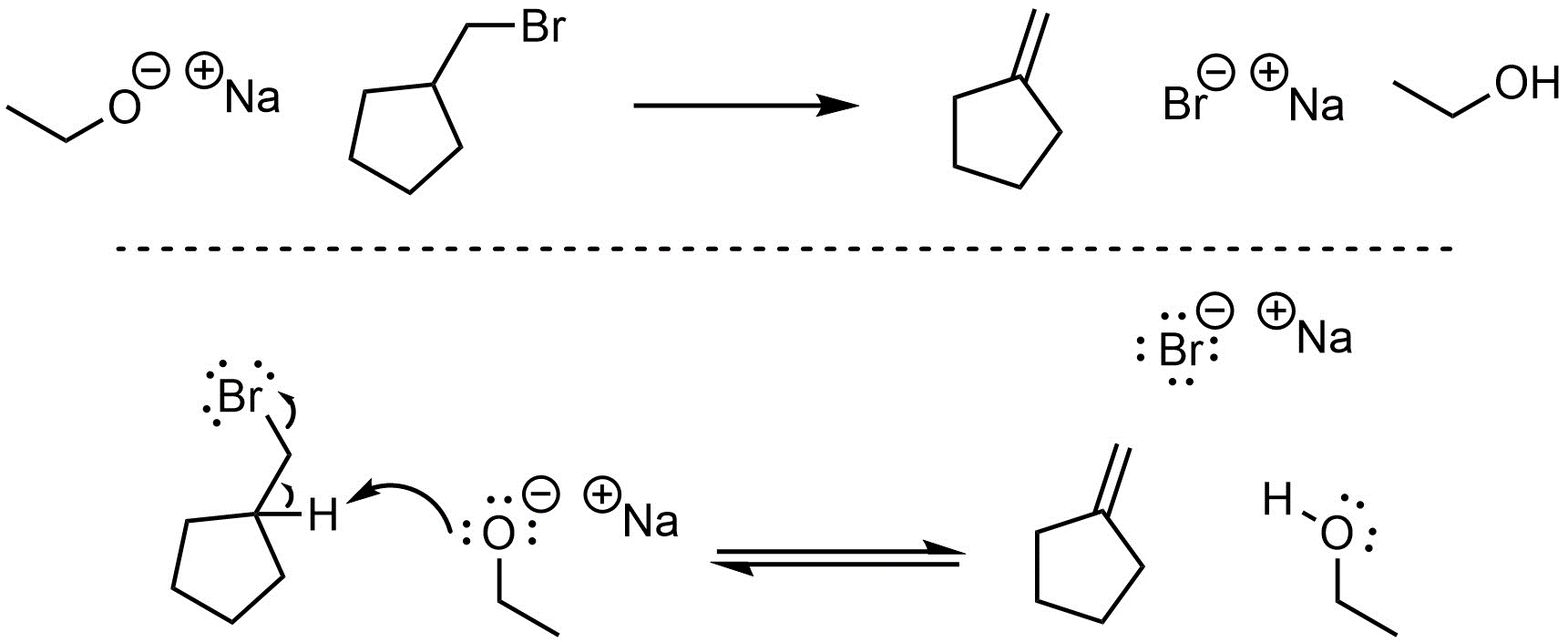
Q12.1: Deep, Eli, and Fatma want to practice elimination reactions. They performed two (below) and generated Compounds B and E. They now want to selectively make Compounds C and F using the same starting materials. Write a brief discussion (small sets of bullet points) rationalizing if it is possible and how it could (or could not) be achieved for each. You may assume reactions would proceed as discussed in the textbook and that Deep, Eli, and Fatma know what they are doing.
Note: This question is meant to be semi-challenging and force you to think critically about multiple factors for both cases; considering what mechanism(s) is/are possible based on the results is only the first step.

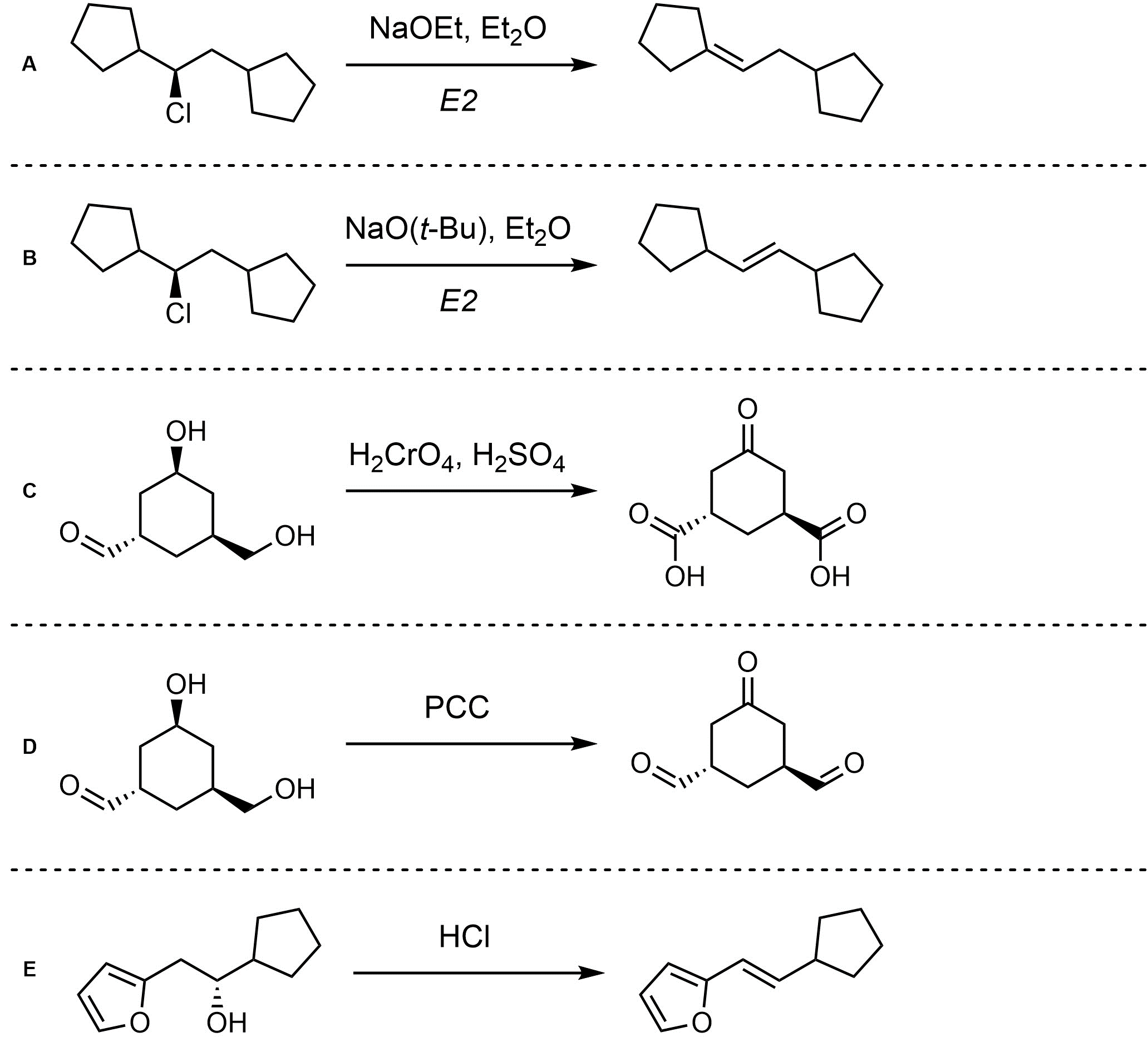
Q12.2: Assume each of the following is an elimination (or elimination-like oxidation) reaction. For each reaction draw the expected major organic product(s). The products must be drawn as line-angle structures. Clearly indicate the appropriate stereochemistry using hashed and wedged bonds where necessary. If the product(s) is/are formed as more than one diastereomer, draw all diastereomers formed. If the product(s) is/are formed as a racemate (racemic mixture) draw only one enantiomer and write the word “racemic” beneath/beside it. Do not write “racemic” if it does not apply.
Note: Remember that it is common but not required to indicate which mechanism is taking place in elimination reactions.
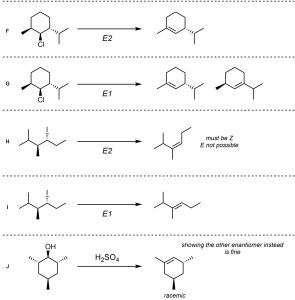

Q12.3: A reaction equation is given below. Propose a reasonable mechanism for the reaction. Show all necessary intermediates, curved arrows, lone pairs, and formal charges.
Must show E2 mechanism. E1 is incorrect. See Section 12.4.