11.4. Determining if a Reaction Follows an SN2 or SN1 Mechanism
It is usually possible to predict whether a nucleophilic substitution reaction will follow an SN1 or an SN2 mechanism. The primary consequence of this is whether or not the product is formed in a stereospecific way (Scheme 11.16).
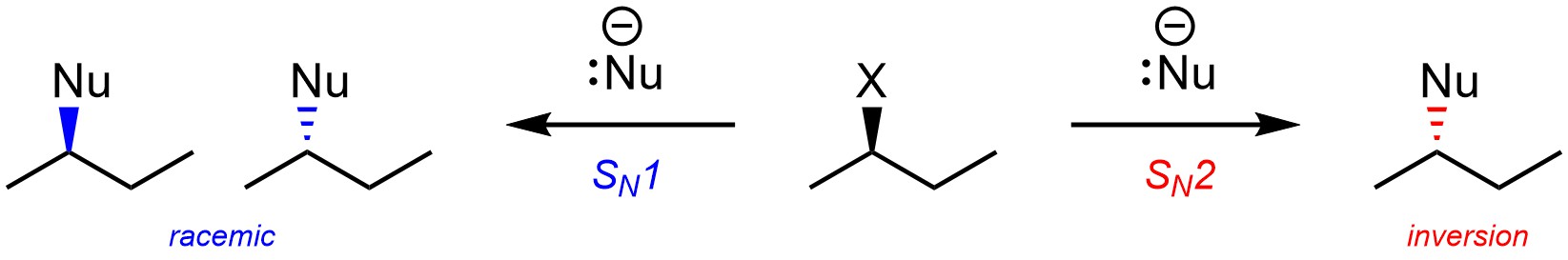
Scheme 11.16 – Example Comparing Stereochemical Outcomes from Nucleophilic Substitution Following an SN1 or SN2 Mechanism.
11.4.1. Reactivity as a Continuum
The two mechanisms, SN1 and SN2, are distinct. Most nucleophilic substitution reactions follow exclusively one or the other mechanism. However, it is possible for the two reaction pathways to have similar activation energies for some compounds. In these rare cases some of the molecules follow the SN1 mechanism and some follow the SN2 mechanism. Even if the starting material was enantiopure the product is not racemic, nor is it perfectly inverted. Instead it is formed as a mixture, with an excess of one of the two absolute configurations at the new stereocentre. At an introductory level it is not possible to accurately predict the stereochemical outcome of these reactions. It is important only to recognize that it is possible for this to occur, understand why it might occur, and recognize what the effect on stereochemistry would be.
11.4.2. Structure of the Electrophile
By far the most important factor in determining which mechanism will be followed is the structure of the electrophile. In all cases the carbocation intermediate that would form if the reaction proceeded through an SN1 mechanism is gauged to determine how likely it is to form, and how stable it would be.
If the carbocation that would be formed has resonance stabilization from a heteroatom with a lone pair the nucleophilic substitution reaction will proceed through an SN1 mechanism (Figure 11.6). The effects of resonance stabilization from π bonds are significantly more complex and cannot be easily predicted at an introductory level. For these cases determining which nucleophilic substitution mechanism is favoured is not required. Students should still be able to predict and draw the product(s) of these reactions.
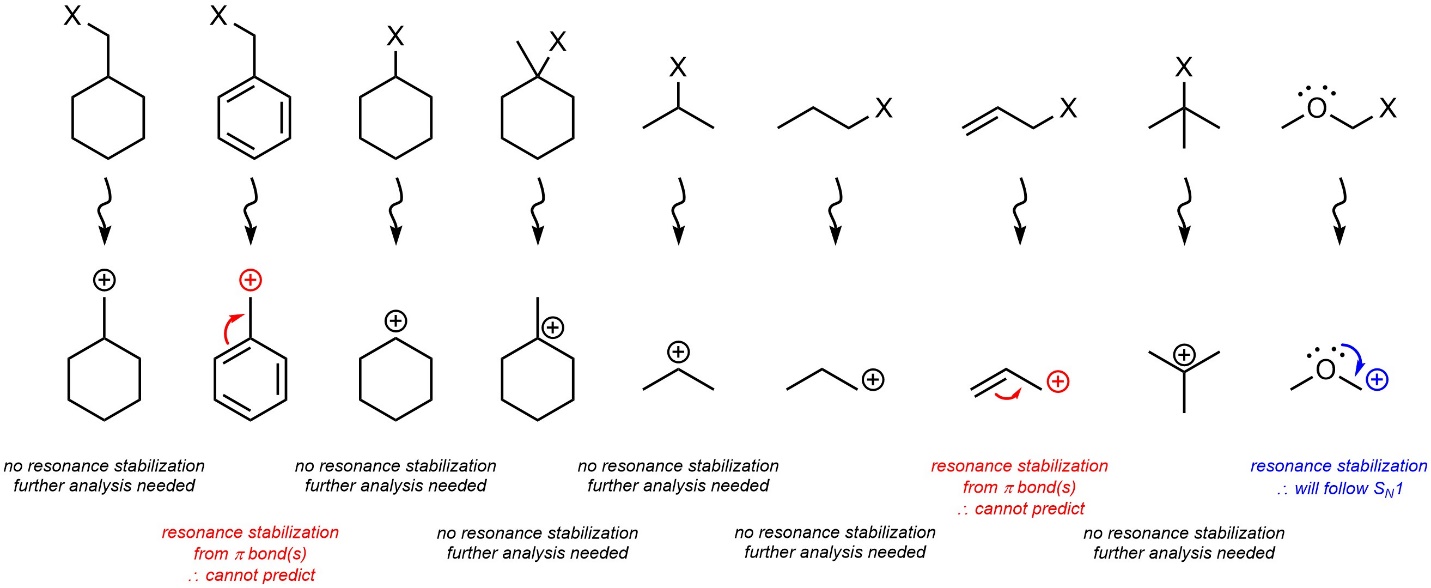
Figure 11.6 – Resonance Stabilization of the Possible Carbocation Intermediate with Lone Pairs Favours SN1 Mechanisms.
In the absence of resonance stabilization: if the carbocation that would be formed is tertiary the nucleophilic substitution reaction will proceed through an SN1 mechanism; if the carbocation that would be formed is primary the nucleophilic substitution reaction will proceed through an SN2 mechanism. If carbocation that would be formed is secondary, then it may proceed through an SN1 or SN2 mechanism and further analysis is needed (see Section 11.4.3.; Figure 11.7).
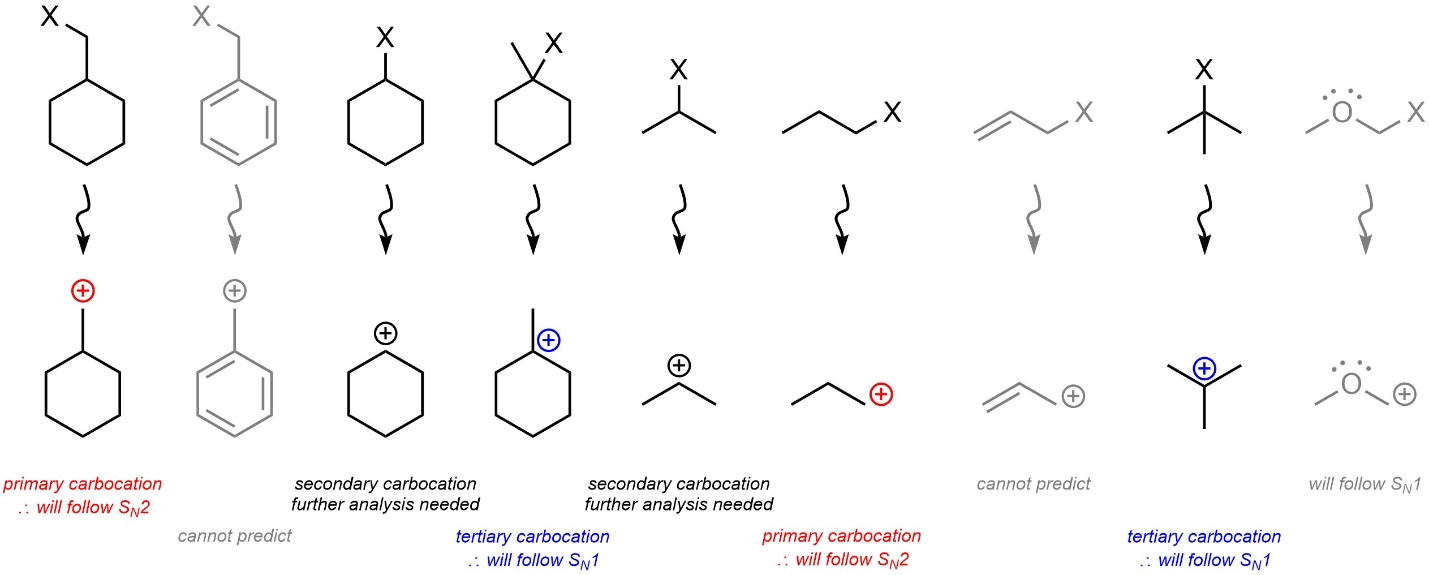
Figure 11.7 – Primary vs. Secondary vs. Tertiary Carbocation Intermediate Effects on SN1 vs. SN2 Mechanisms.
Other factors such as a large number of steric interactions, induction, and/or geometric constraints may affect the reaction mechanism. This has important consequences for biology and chemical synthesis but is too complex a topic to explore in-depth at an introductory level.
11.4.3. Solvent Effects
If the carbocation that would be formed is secondary, then it may proceed through an SN1 or SN2 mechanism. The specific nature of the nucleophile can be analyzed to help determine which pathway will be followed. However, it is simpler to analyze the nature of the solvent. Solvent effects should only be considered if the electrophile has no resonance stabilization and is secondary. Structural effects from the electrophile (see Section 11.4.2.) are much stronger forces for determining the mechanism of nucleophilic substitution reactions.
Recall that solvents may be classed as either protic or aprotic depending on whether they can or cannot donate a hydrogen bond (see Section 2.4.2.1.). Recall that this requires a polar bond to a hydrogen, typically an N-H or O-H bond.
Polar solvents slightly stabilize carbocations by coordinating with them. Although the coordinating atoms are not actually anionic, it is useful to think of this as similar to an ionic bond (Figure 11.8). Because a carbocation is formed in the SN1 mechanism but not in the SN2 mechanism, this stabilization only lowers the activation energy for the SN1 pathway, making it faster. This is true in both protic and aprotic solvents.
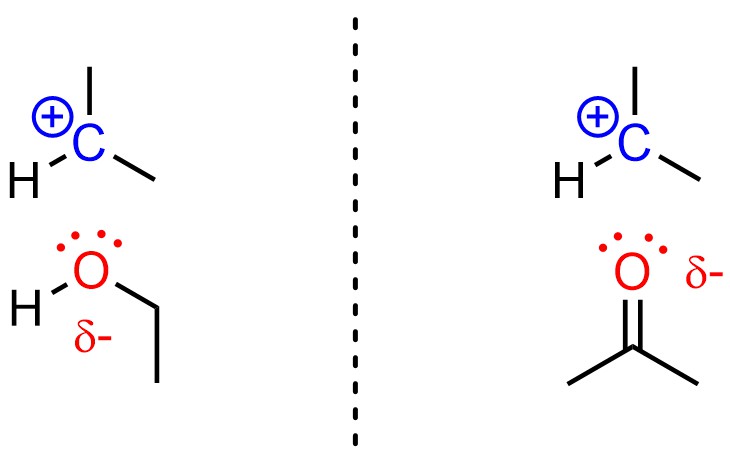
Figure 11.8 – Examples of a Protic Solvent (Ethanol) and an Aprotic Solvent (Acetone) Stabilizing a Carbocation.
Polar Protic solvents favour SN1 mechanisms. In addition to the above effect, protic solvents may hydrogen bond with anions and nucleophiles. This stabilizes the anion (from the leaving group) that is formed when the carbocation is made, further lowering the activation energy of SN1 mechanisms (Figure 11.9).
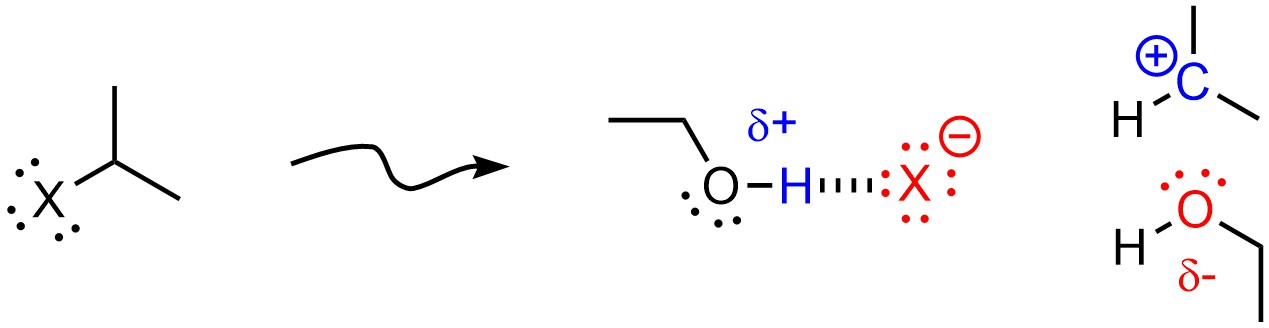
Figure 11.9 – Example of a Protic Solvent (Ethanol) Stabilizing an Anionic Leaving Group and a Carbocation.
The stabilization also slightly decreases the strength of nucleophiles (Figure 11.10). Because nucleophilicity only affects the rate of SN2 reactions, making it less nucleophilic only makes SN2 pathways slower. All three effects favour the SN1 mechanism.
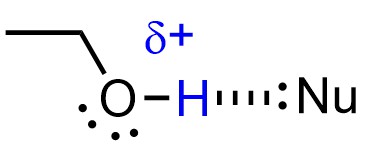
Figure 11.10 – Example of a Protic Solvent (Ethanol) Stabilizing a Nucleophile by Hydrogen Bonding.
Polar Aprotic solvents favour SN2 mechanisms. The exact reason for this is controversial. The currently accepted theory is that aprotic solvents slightly destabilize anions and nucleophiles through coordination; the same interaction that stabilizes cations destabilizes anions. Because the rate-determining step of the SN1 mechanism usually involves formation of an anion (as the leaving group leaves) this raises the activation energy for the SN1 pathway, making it slower. This counteracts the stabilization of the carbocation from the solvent being polar (Figure 11.11).
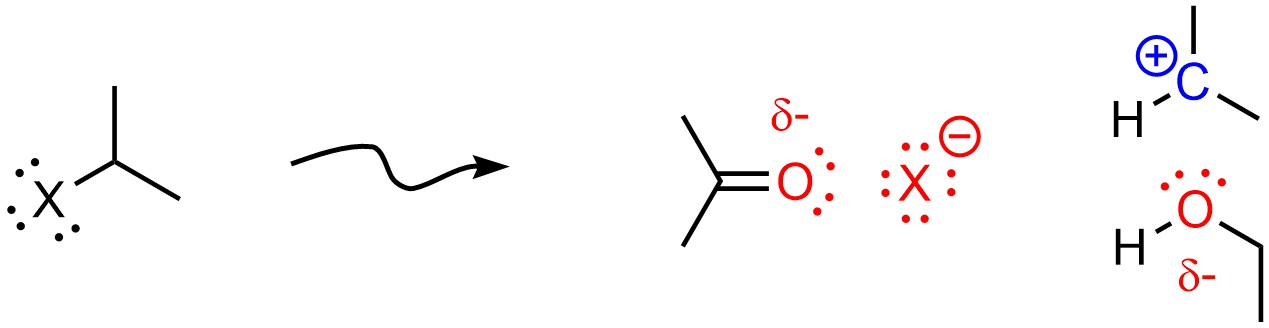
Figure 11.11 – Example of an Aprotic Solvent (Acetone) Destabilizing an Anionic Leaving Group and Stabilizing a Carbocation.
The destabilization also slightly increases the strength of nucleophiles (Figure 11.12). Because nucleophilicity only affects the rate of SN2 reactions, making it more nucleophilic only makes SN2 pathways faster. These two destabilizing interactions outweigh the stabilizing one, overall favouring the SN2 mechanism.
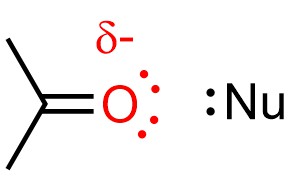
Figure 11.12 – Example of an Aprotic Solvent (Acetone) Destabilizing a Nucleophile.
Non-Polar solvents are not appropriate for either type of nucleophilic substitution reaction. Both mechanisms require polar solvents. With a non-polar solvent, at least one of the starting materials, intermediates, or products would not be soluble.

