1.8. Other Representations of Molecules
1.8.1. Condensed Structures
A condensed formula or structure is made up of the elemental symbols. Condensed structural formulas show the order of atoms like a Lewis Structure but are written in a single line to save space and make it more convenient and faster to write out. The order of the atoms suggests the connectivity in the molecule. Condensed structural formulas are also helpful when showing that a group of atoms is connected to a single atom in a molecule. When this happens, parentheses are used around the group of atoms to show they are together. Also, if more than one of the same substituent is attached to a given atom, it is shown with a subscript number. An example is CH4, which represents four hydrogens attached to the same carbon. Condensed formulas can be read from either direction and H3C is the same as CH3, although the latter is more common (Figure 1.40). Condensed structures work best for small, simple molecules where there is little ambiguity between possible connection points.
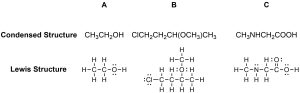
Figure 1.40 – Condensed and Lewis Structures for Three Compounds.
This style is largely a holdover from the typewriter era, when it was much easier to include a line of text than to add a drawing for every molecule being discussed. Nonetheless they continue to be used for many simple compounds.
1.8.2. Line-Angle // Bond-Line // Skeletal Structures
Line-angle formulas are used to write carbon and hydrogen atoms more efficiently by replacing the letter “C” with lines. A carbon is present wherever a line intersects another line and at the ends of lines (Figure 1.41). Hydrogens are omitted but are assumed to be present to complete each of carbon’s four bonds. Hydrogens that are attached to elements other than carbon are shown. Atom labels for all other elements are shown.
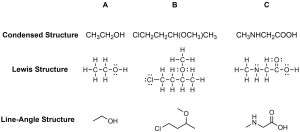
Figure 1.41 – Condensed and Lewis Structures for Three Compounds.
Lone pair electrons are sometimes omitted, but they can be included without issue (Figure 1.42a). Normally the angle between lines is 120° to approximate tetrahedral and trigonal planar geometry, though angles of 180° are used when linear geometries are present (Figure 1.42b). If the three-dimensional arrangement is known (absolute configuration, see Chapter 4) it may be included using hashed/wedged bonds, though hydrogens attached to carbons are still omitted (Figure 1.42c).
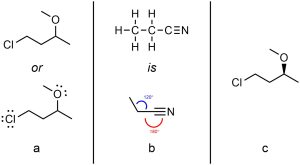
Figure 1.42 – Other Considerations When Drawing Line-Angle Structures.
1.8.3. Three-Dimensional Representations
There are numerous other ways of depicting molecules in three-dimensions. In organic chemistry a common approach is to use differently coloured ellipsoids (spheres) to represent different atoms (Figure 1.43). Bonds are typically represented using lines or cylinders connecting the spheres. These models are sometimes called Ball-and-Stick and are meant to approximate seeing a molecular model. This approach is very useful for showing three-dimensional features. These images are generated using computer software and are not meant for students to recreate, but it is important to be able to recognize what is being depicted. Most representations use the same colours to refer to the same elements. For example, the computer-generated image coloured the oxygen atom as red, and a standard molecular model kit will have red balls for oxygen atoms. However, these are only conventions and not rules; the computer-generated image coloured the carbon atoms grey, but a standard molecular model kit will have black balls for carbon atoms.

Figure 1.43 – Using Ball-and-Stick Models to Show Molecules in Three-Dimensions.

