7.3. Simple Nucleophilic Attacks on Carbonyls
Carbonyl-containing functional groups represent a simple model for nucleophile-electrophile reactions without introducing a large amount of terminology.
7.3.1. Examples
Nucleophiles form new bonds by donating a pair of electrons to an electrophilic atom. A nucleophilic attack from methoxide onto acetone forms a new C-O bond (Scheme 7.1). Notice that the overall charge does not change during the reaction; the starting materials have the same overall charge as the products.
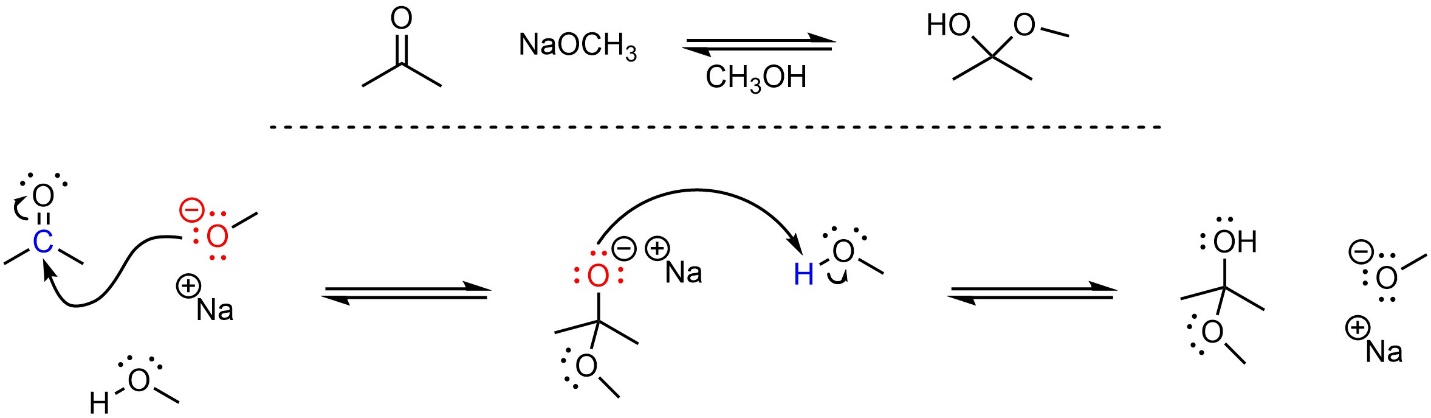
Scheme 7.1 – Reaction Mechanism for Sodium Methoxide Attacking Acetone in Methanol.
If there are proton sources (acids) around they will neutralize the charge. This can come from the solvent or from an acid added after the reaction is complete (Scheme 7.2). Adding an acid (or base) at the end of a reaction to neutralize the charge, form the desired product, and consume any excess reagent is referred to as quenching the reaction.
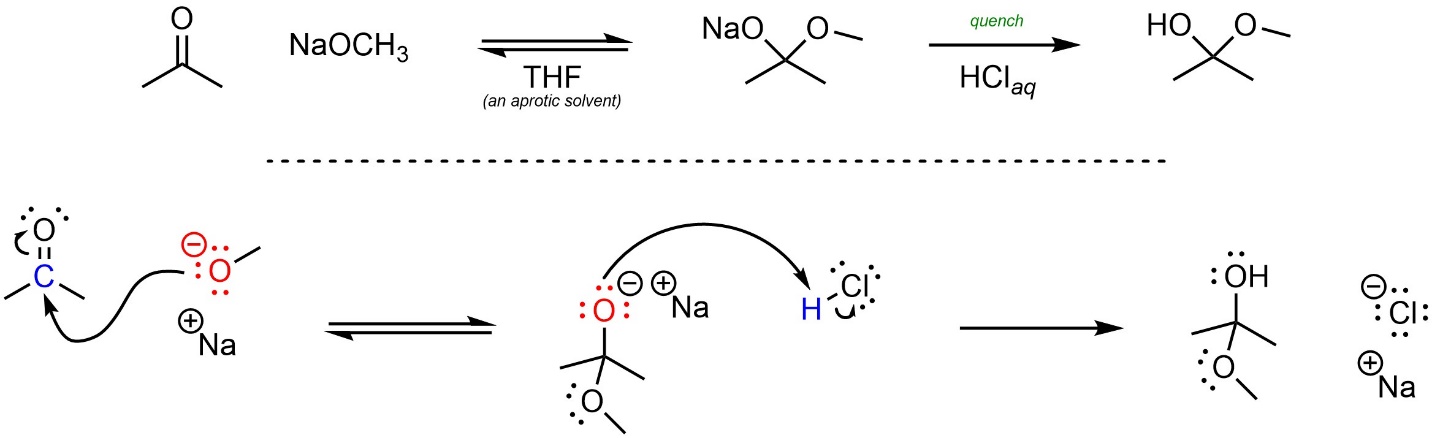
Scheme 7.2 – Reaction Mechanism for Sodium Methoxide Attacking Acetone Followed by an Acid Quench.
It is very important to remember that, despite often drawing them as such, cations and anions do not exist without a counterion. Recall that, if possible, one should always show both the anion and the cation when drawing chemical reactions or mechanisms. This avoids situations where a molecule “disappears” at one step but is needed for a later step (see Section 5.3.4).
Contrast the attack of methoxide on acetone with the attack of methanol on acetone (Scheme 7.3). Note that the second step is an oversimplification common to introductory texts; a more accurate mechanism for the second step involves another equivalent of the alcohol (bottom). Drawing either is acceptable at an introductory level.
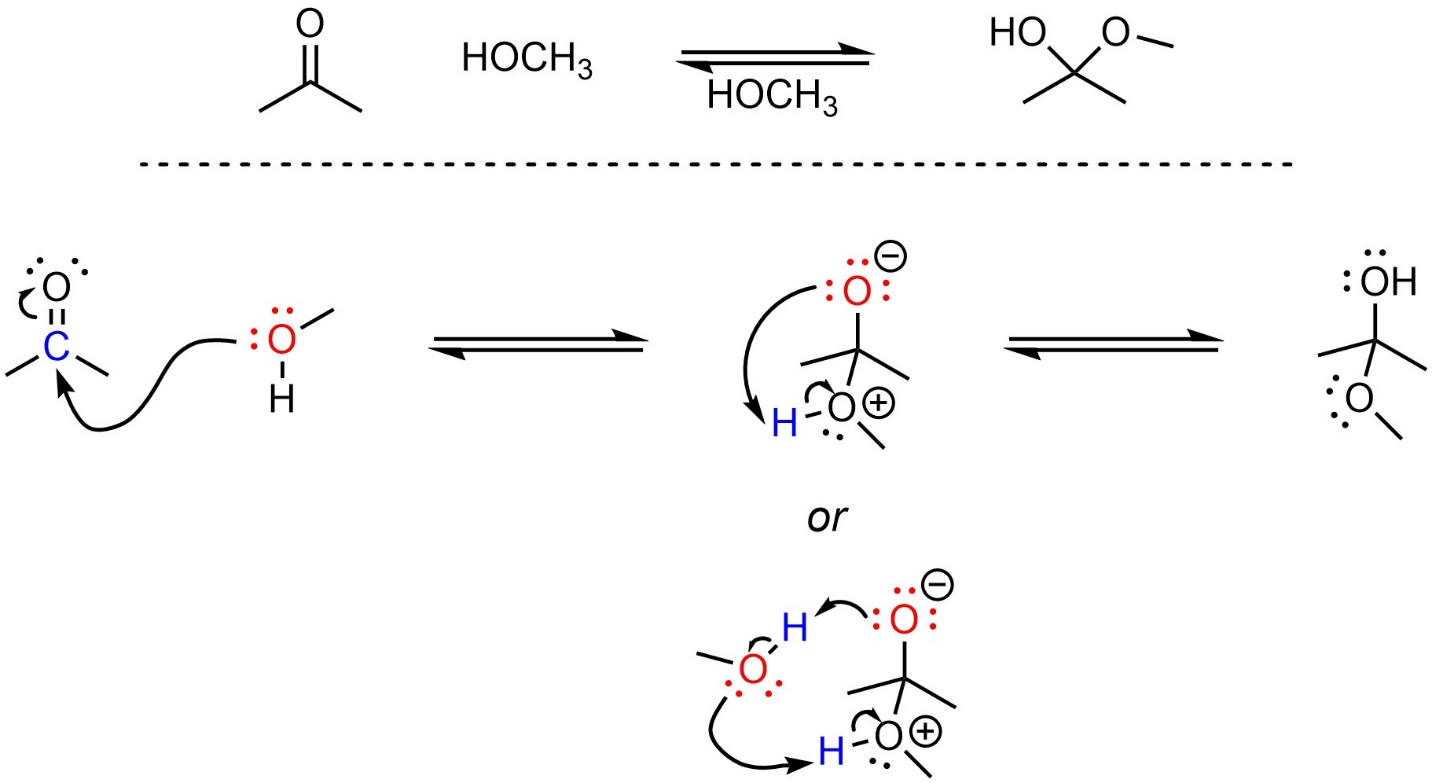
Scheme 7.3 – Reaction Mechanism for Methanol Attacking Acetone.
7.3.2. Hydride Attacks
The smallest nucleophile is hydride (H–). Hydride on its own, such as in sodium hydride (NaH) or potassium hydride (KH), could act as a nucleophile but instead it almost always acts as a base. This irreversibly forms hydrogen gas (H-H) and consumes the potential nucleophile. If nucleophilic hydride is desired a hydride source such as borohydride (–BH4) or aluminum hydride (–AlH4) is used instead.
Although both the boron and aluminum atoms in the above anions have negative formal charges, they are not nucleophilic; the B-H and Al-H bonds are polar with hydrogen being more electronegative than either boron or aluminum (see Figure 7.5). The hydrogen atoms have excess electron density and are nucleophilic, acting as hydrides.
The most common borohydride source is sodium borohydride, which can be used to attack carbonyl-containing functional groups with hydride (Scheme 7.4). Either protic or aprotic solvents may be used. An added benefit of this reaction is that the side-product, borane (BH3), can react with alkoxides to generate another hydride source. This means that each equivalent of borohydride can deliver four equivalents of hydride; one molecule of NaBH4 can add H– to four carbonyls.
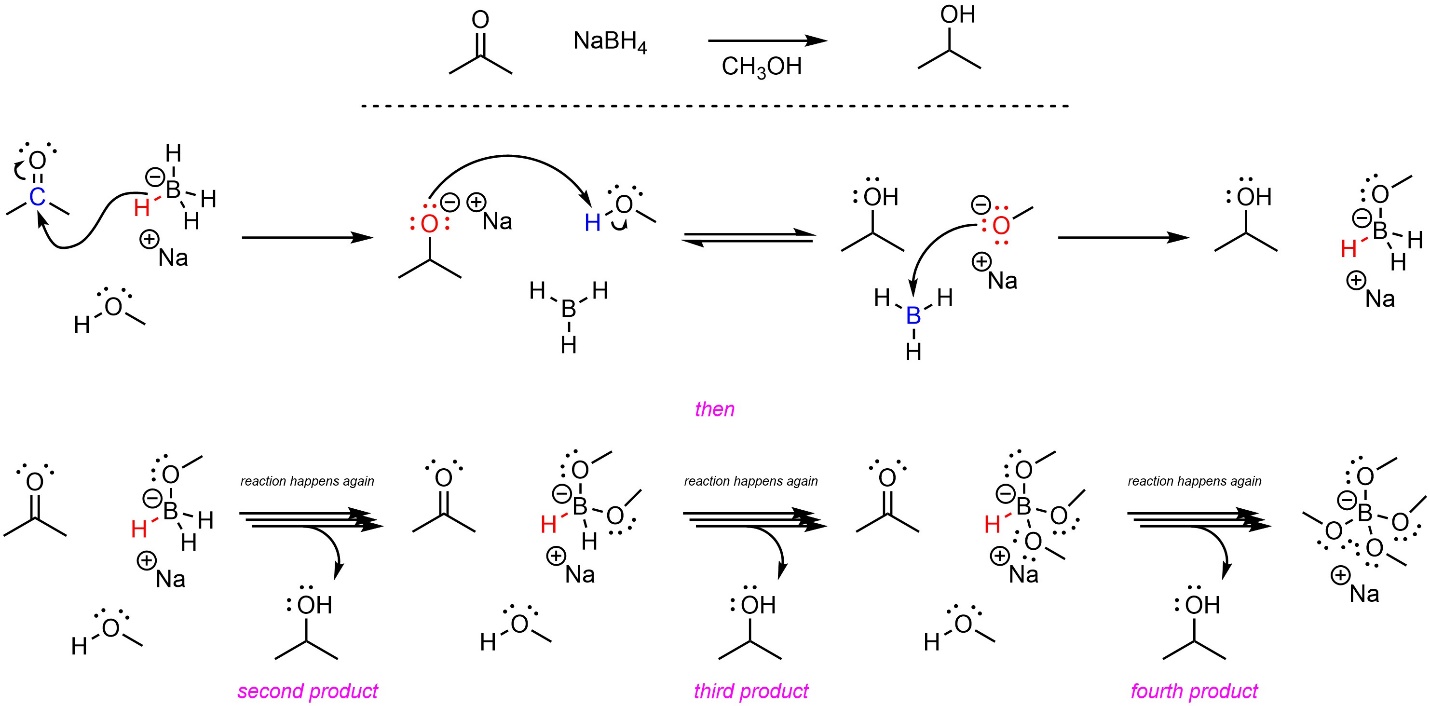
Scheme 7.4 – Reaction Mechanism for Borohydride Attacking Acetone.
The most common aluminum hydride source is lithium aluminum hydride (sometimes abbreviated as LAH), which can also be used to attack carbonyl-containing functional groups (Scheme 7.5). Because there is a (much) larger difference in electronegativity, the hydrides attached to aluminum are much more nucleophilic than those in borohydride. As a result, only aprotic solvents may be used. As with sodium borohydride, an added benefit of this reaction is that the intermediate formed is another hydride source. This means that each equivalent of aluminum can deliver four equivalents of hydride; one molecule of LiAlH4 can add H– to four carbonyls.
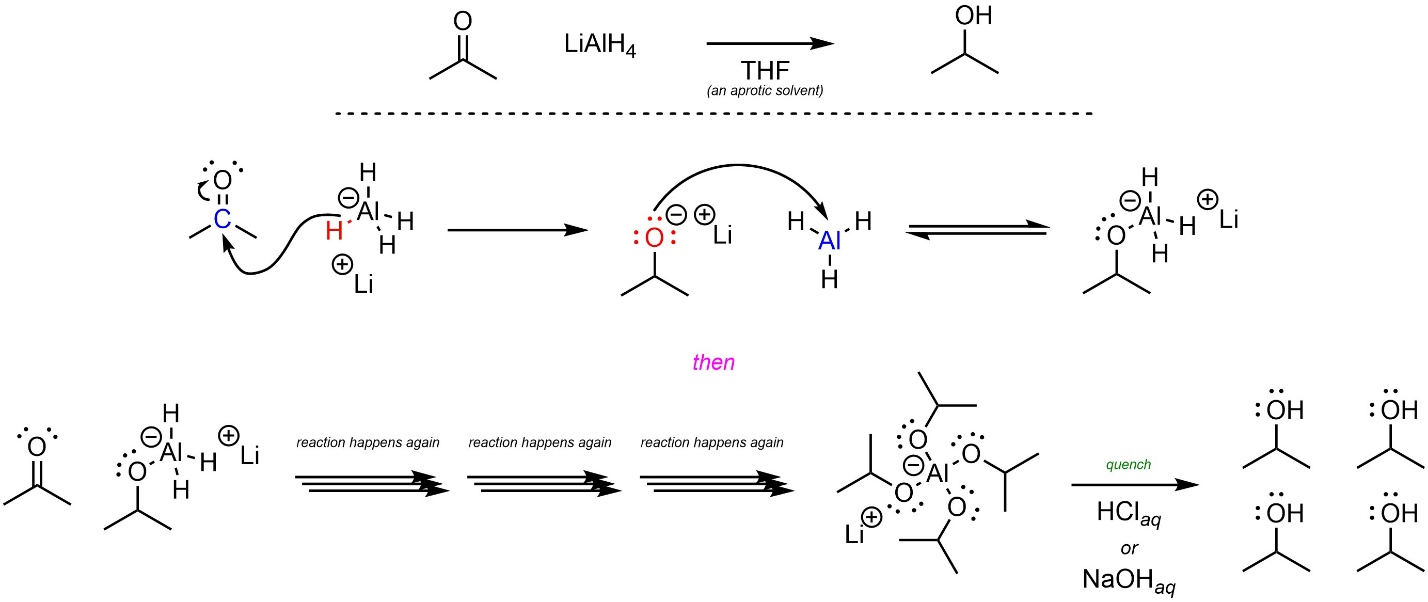
Scheme 7.5 – Reaction Mechanism for Aluminum Hydride Attacking Acetone.
Because aprotic solvents are used the product is not formed directly; this reaction must be quenched using a strong acid (or strong base) to break the O-Al bonds and generate the final product. The overall reaction is often depicted as two steps to indicate that this is required (Scheme 7.6). The mechanisms for either quenching step are simple but would require additional information to discuss.
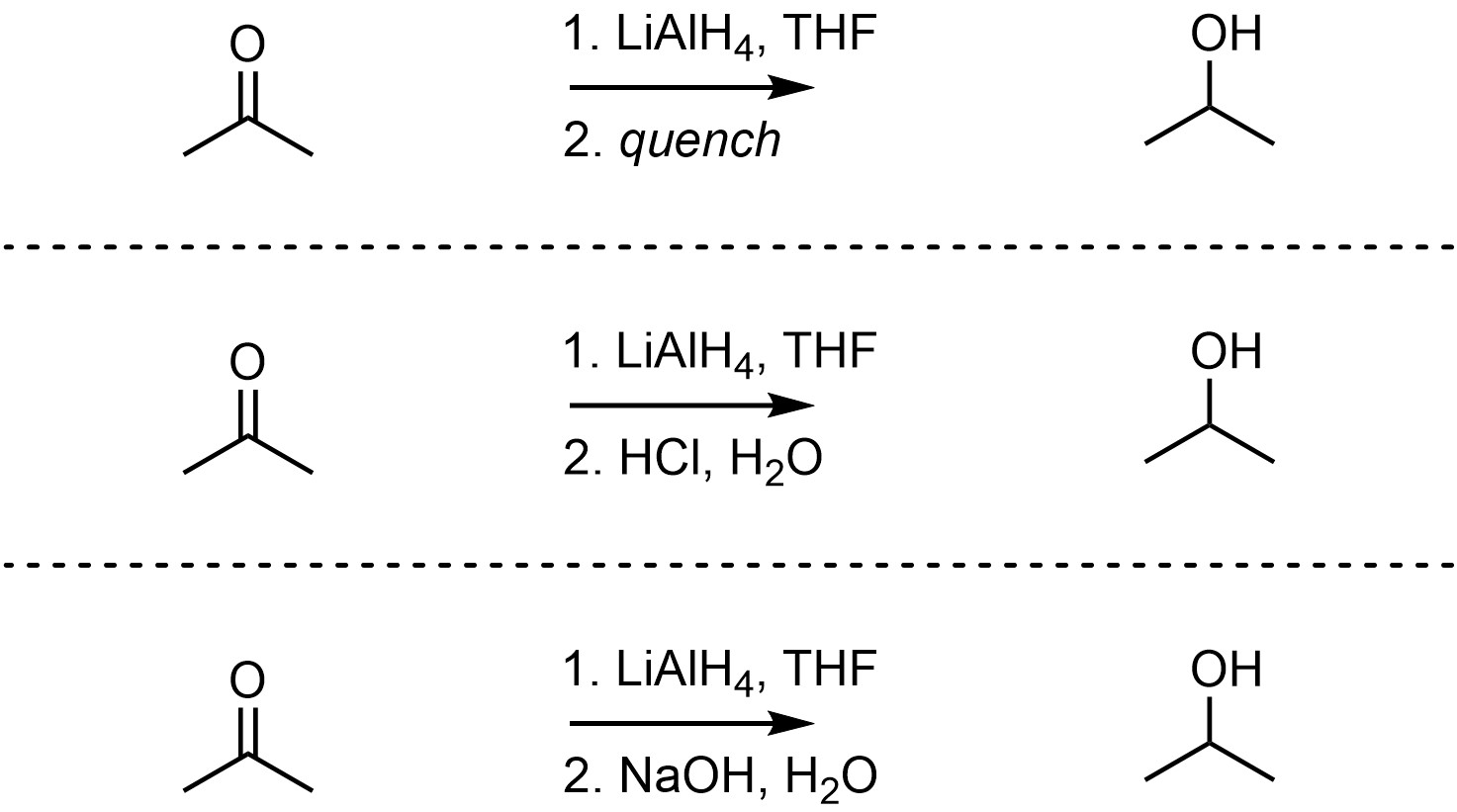
Scheme 7.6 – Examples of Ways of Depicting Overall Reactions for Aluminum Hydride Reactions.
In both borohydride and aluminum hydride reductions, it is more common to simply draw the mechanism for the reduction of a single equivalent of carbonyl and understand that a further three reductions are possible. At an introductory level this is acceptable.

