11.3. Substitution Reactions: SN1 Reactions
Nucleophilic substitution reactions (sometimes called Displacement reactions) can occur with one or two steps in the mechanism. With two steps in the mechanism, SN1 reactions are different from acid-base reactions. They are not stereospecific and follow a slightly modified mechanism.
11.3.1. Mechanism
Nucleophilic substitution reactions that follow the SN1 mechanism have two steps (Scheme 11.14). The leaving group leaves, generating a carbocation. This greatly increases its electrophilicity. Then, the nucleophile attacks the carbocation (electrophile), creating a new bond. This occurs sequentially. The new bond forms after the old bond breaks.
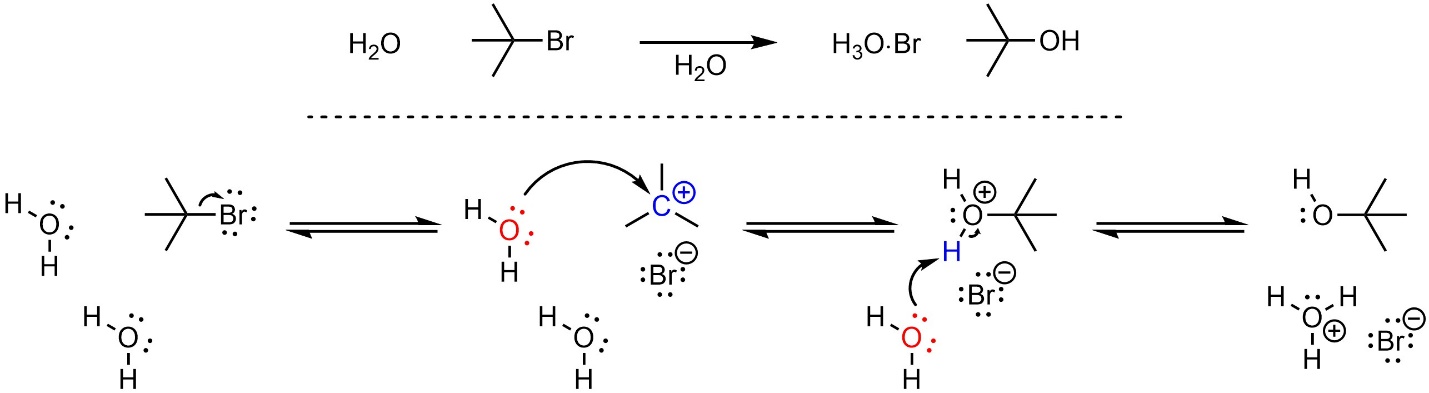
Scheme 11.14 – Reaction Mechanism for SN1 Nucleophilic Substitution of 2-Bromo-2-methylpropane with Water.
Depending on the specific reaction that is occurring there may be additional steps before or after the substitution step. For example, the nucleophile or electrophile may be activated prior to the substitution, or there may be a protonation or deprotonation after the nucleophile attacks the electrophile. These extra steps do not change the classification of the mechanism for the substitution step; if the old bond breaks before the new bond forms, it is still classed as an SN1 nucleophilic substitution reaction.
11.3.2. Reaction Coordinate and Rate-Determining Step
The first step, carbocation formation, requires a large amount of energy and is the rate-determining step (Figure 11.3). The overall reaction is slightly exergonic.
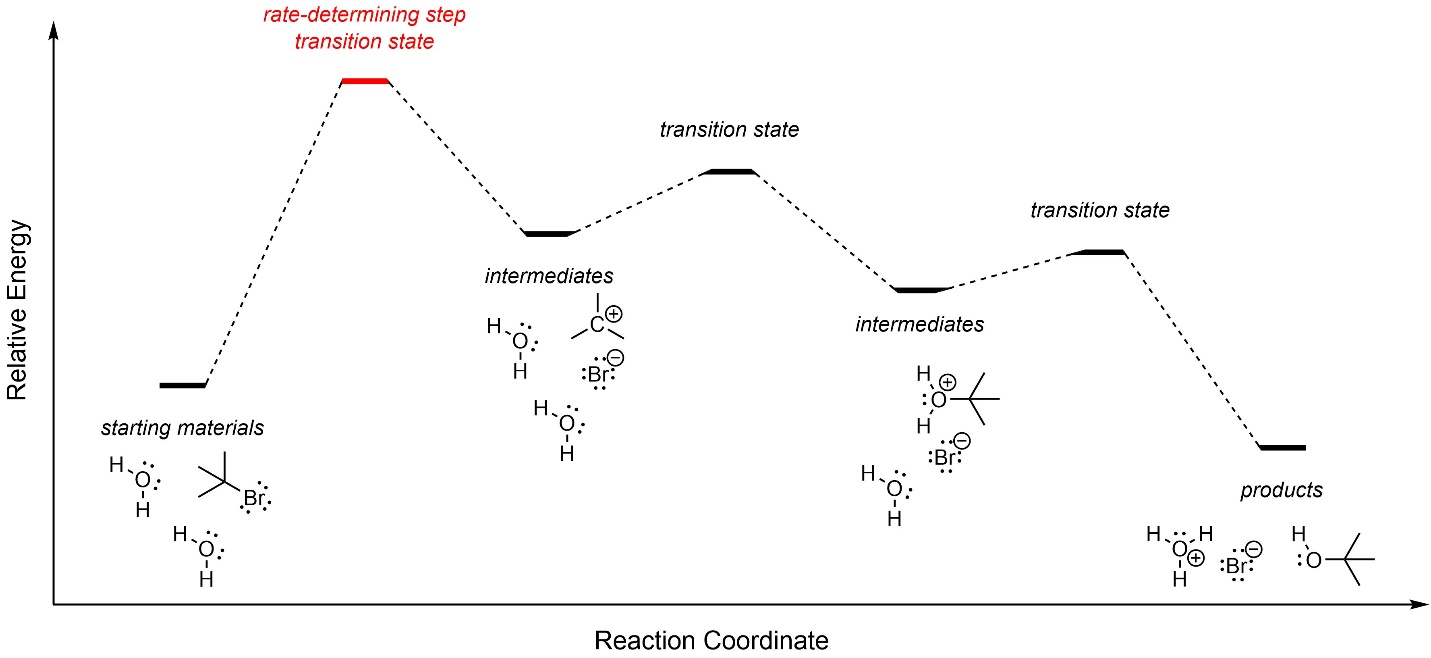
Figure 11.3 – Reaction Coordinate for SN1 Nucleophilic Substitution of 2-Bromo-2-methylpropane with Water.
11.3.3. Stereoselectivity
Recall that carbocations are sp2 hybridized, with the p orbital being unoccupied. The empty p orbital which makes up the electrophilic site is located both above and below the carbon (Figure 11.4). As a result, the nucleophile may approach and attack a carbocation from either side. Because it is now trigonal planar, even if the carbon was previously a stereocentre it is no longer one.
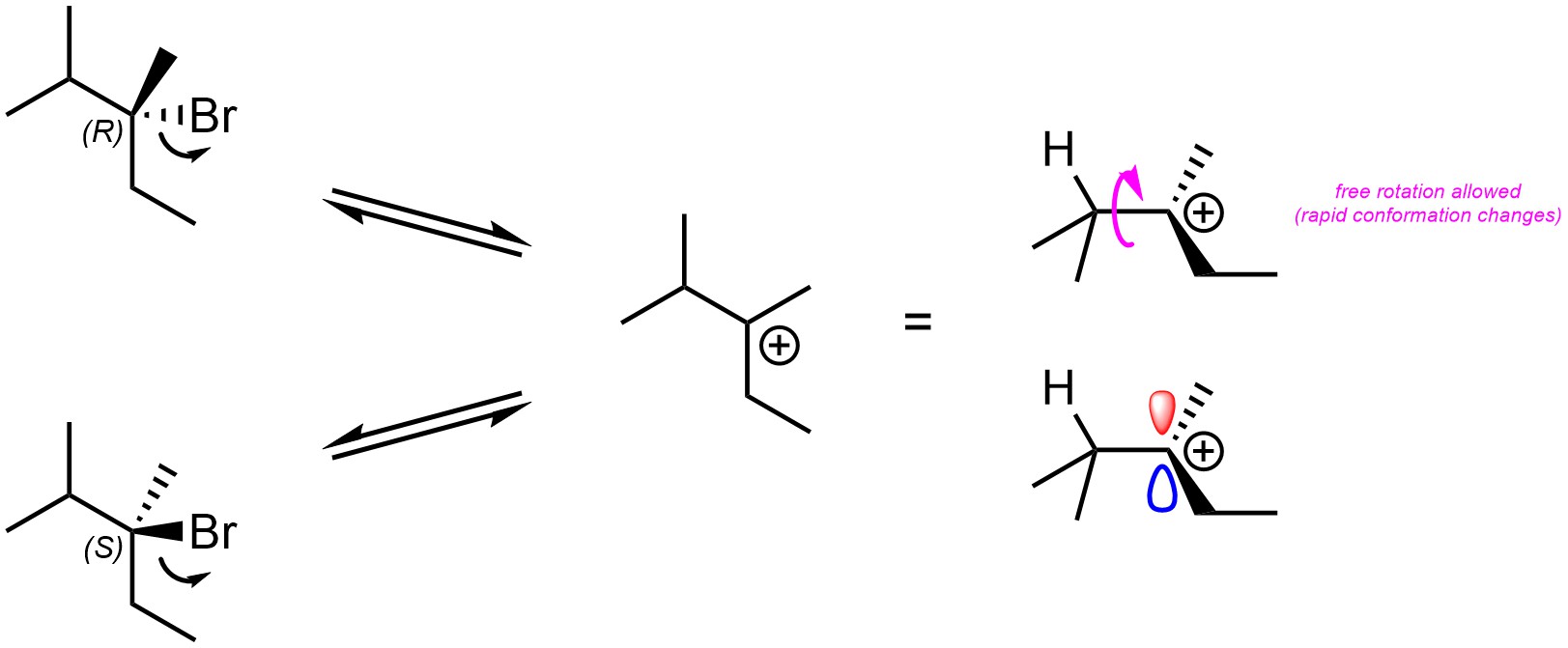
Figure 11.4 – Trigonal Planar Geometry of Carbocations and Example of Formation of a Carbocation Resulting in Destruction of a Stereocentre.
Stereochemical considerations for SN1 reactions are very similar to those for attacks from π bonds on electrophiles (see Section 8.5.3.). Assuming there are no stereocentres in the intermediate, the second step (nucleophile attacks carbocation) will not be stereoselective as attacking the top of the carbocation is sterically and electronically equivalent to attacking the bottom of the carbocation (Figure 11.5). If a new stereocentre is formed during this step, it will be formed as an equal mixture of both (R) and (S). This step is not stereoselective.
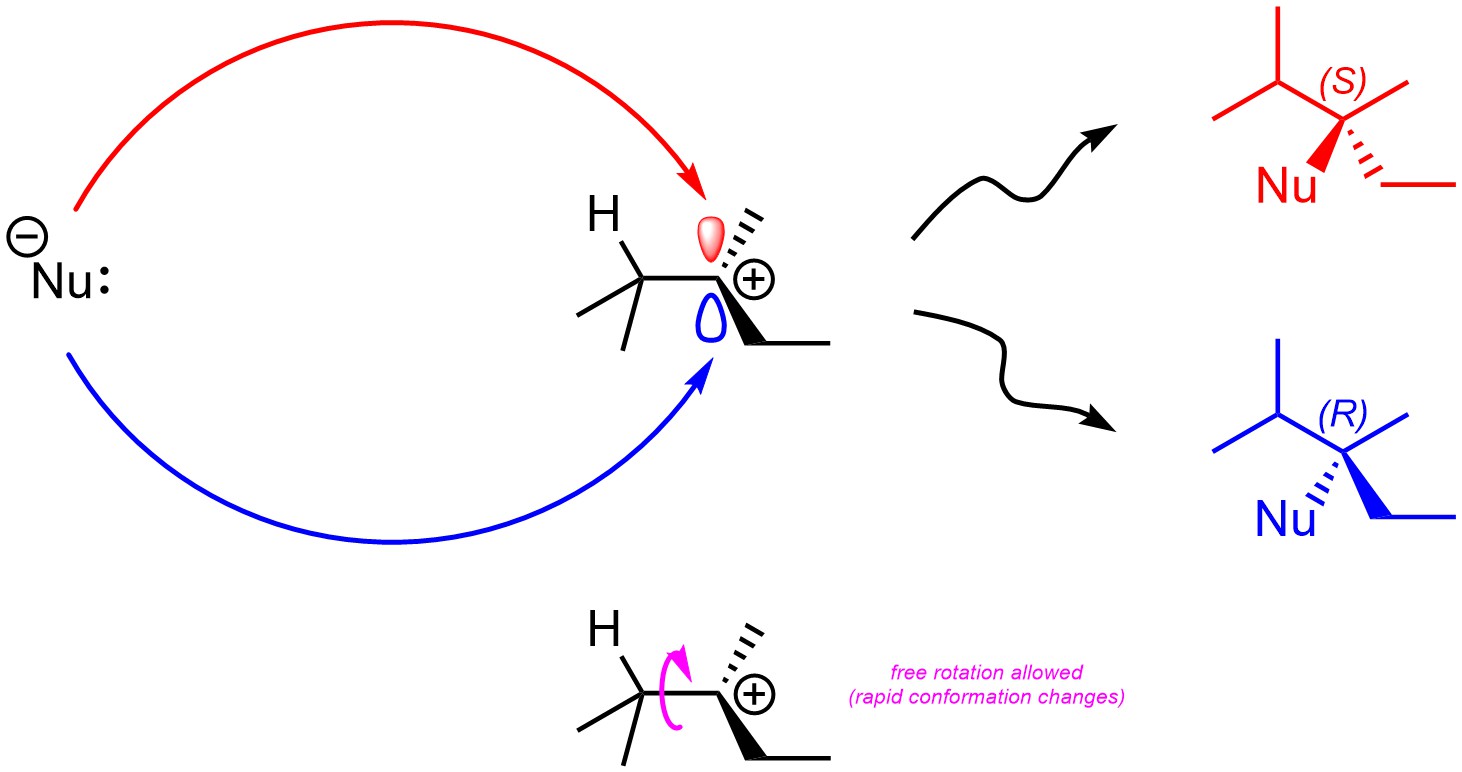
Figure 11.5 – Comparison of Products from Nucleophilic Attacks on the Top and Bottom Sides of 2,3-Dimethylpentan-3-ylium.
In either step, if another stereocentre (or multiple stereocentres) exists then the resulting intermediates/products will be diastereomers. As with nucleophilic attacks on carbonyls (see Section 7.8) or nucleophilic attacks from alkenes (see Section 8.5.3) because there is a steric and/or electronic difference between attacking the top or bottom in these cases, the two stereoisomers are NOT formed equally and the product is a non-one-to-one mixture of diastereomers. In these cases, it is important only to recognize that the product will be formed as a mixture of diastereomers. Predicting which diastereomer should be favoured is not required.
11.3.4. Reaction Rate
Nucleophilic substitution reactions that follow SN1 pathways have two steps in their mechanisms. The “1” in the name refers not to the mechanism but to the reaction’s rate law, which is first-order and dependent on one thing: the electrophile. This is because only the electrophile is involved in the rate-determining step.
Because the reaction rate is not affected by the nucleophile, anything that makes the nucleophile more or less nucleophilic will not increase or decrease the rate of the reaction. Because the reaction rate is affected by the electrophile, anything that makes the electrophile more electrophilic will increase the rate of the reaction. The opposite is also true; weaker electrophiles will make the reaction slower.
11.3.4.1. Effects from the Nucleophile
The strength of the nucleophile will not affect the rate of SN1 reactions. As long as it is nucleophilic the reaction will proceed.
11.3.4.2. Effects from the Electrophile
All of the effects on electrophilicity from leaving group ability for SN2 reactions also apply to SN1 reactions (see Section 11.2.4.2).
Because a carbocation is formed all effects that stabilize carbocations (see Section 8.5.3.) will also affect the rate of SN1 reactions. Carbocations can be stabilized by having resonance delocalization (Scheme 11.15). This is the most important factor that can stabilize a carbocation. Since having adjacent alkyl groups stabilizes cationic charges, in the absence of delocalization tertiary carbocations will be more stable than secondary carbocations, both of which are significantly more stable than primary carbocations.
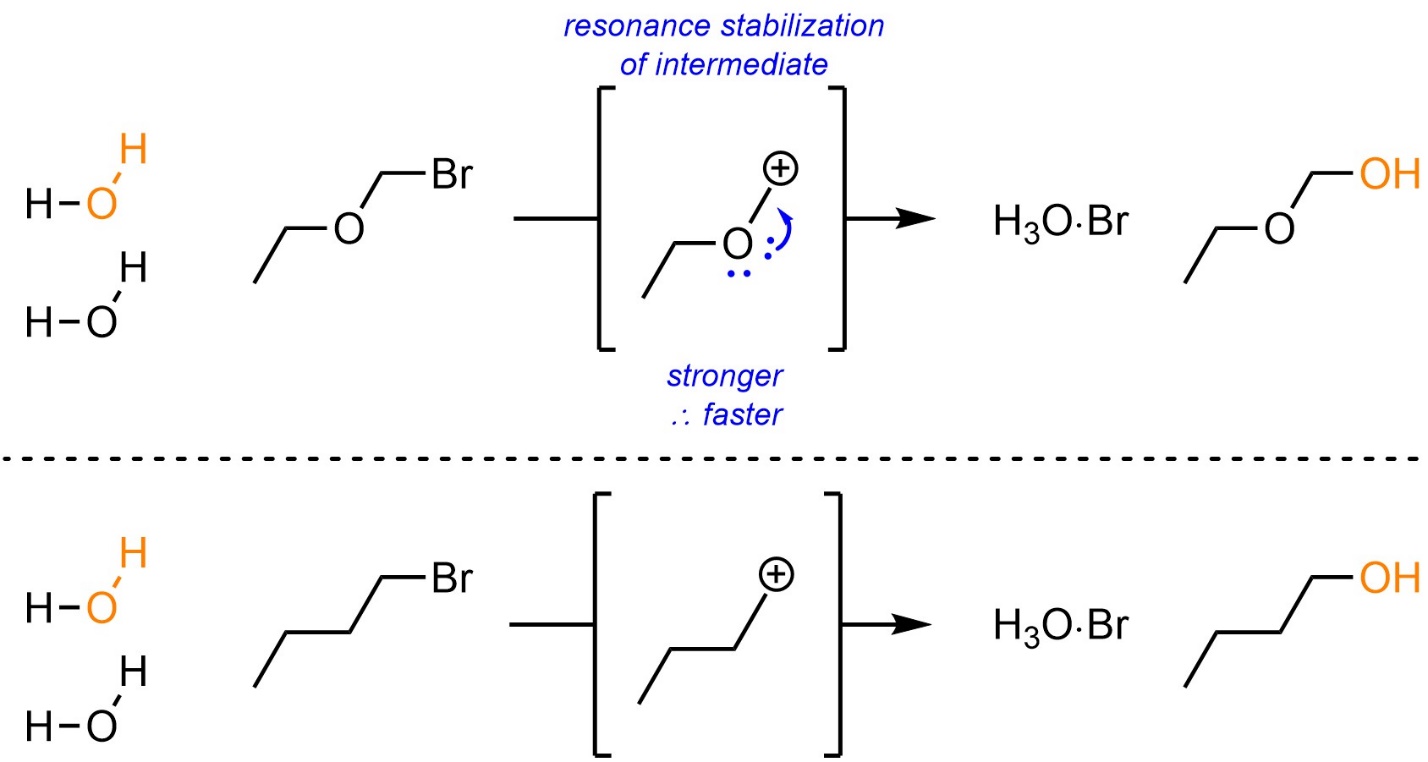
Scheme 11.15 – Example of the Effects of Resonance Stabilization of the Carbocation Intermediate on SN1 Reactions.

