13.1. (Very) Brief Refresher of the Basics
Recall that carbonyls are not functional groups on their own but are part of many functional groups. For example, aldehydes, ketones, carboxylic acids, esters, acid halides, and amides all contain carbonyls (Figure 2.25).
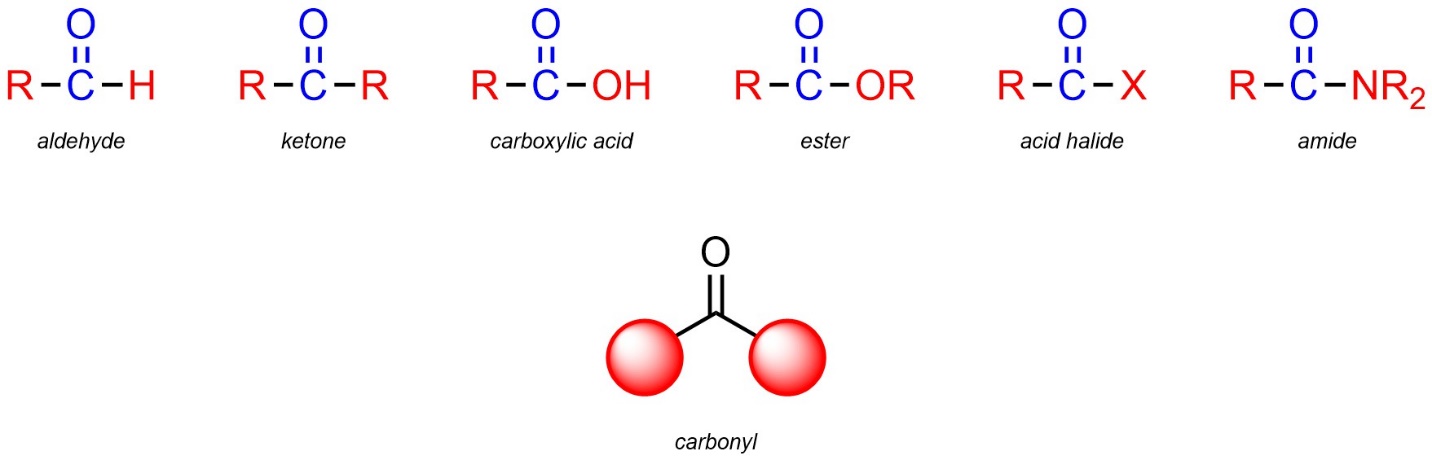
Figure 2.25 – Examples of Carbonyls in Functional Groups.
Because this feature is common to so many groups, it imparts a similar type of reactivity to all groups with it. All carbonyls are electrophilic (see Section 7.3) with the carbon of the carbonyl being the electrophilic atom. This means that functional groups that contain a carbonyl are also electrophiles and are electrophilic at the same place.
Recall that the degree of electrophilicity can vary depending on steric interactions and electronic effects (see Section 7.2.3). For example, aldehydes and ketones have different electrophilicities due to steric interactions. However, an even larger difference exists between them and functional groups with additional resonance stabilization of the electrophilic position (Figure 7.9).
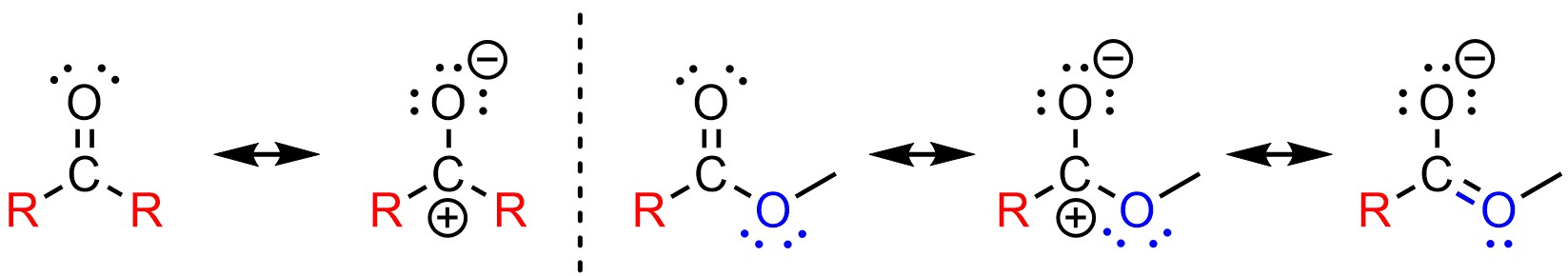
Figure 7.9 – Comparison of Electronic Differences Between Ketones and Esters.
This is generally true for all functional groups that have a heteroatom directly connected to the carbonyl (carboxylic acids, esters, acid halides, amides, etc). As a result, all of these functional groups have similar chemical reactivities to each other that are related to, but different from, the chemical reactivities of aldehydes and ketones.

