10.3. General Form and Mechanism
Electrophilic aromatic substitution reactions all follow the same overall form, where a hydrogen on the aromatic ring is replaced with a new group (Scheme 10.2). It is possible for heteroaromatic rings to undergo SEAr reactions, but these tend to be more complex and are typically omitted from introductory material.
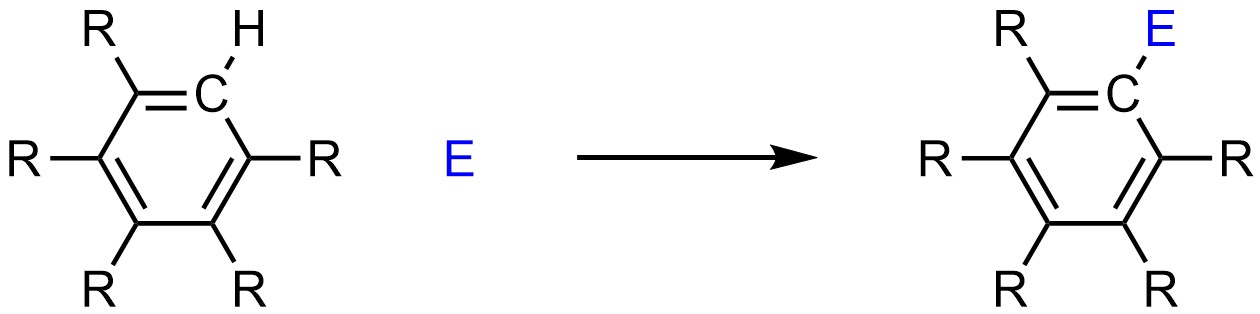
Scheme 10.2 – Generalized Reaction Equation for Electrophilic Aromatic Substitution.
The exact reaction mechanism varies slightly depending on what electrophile is used (Scheme 10.3). First, the electrophile is activated in some way (not shown). The exact way this is done depends on the electrophile. Then, a π bond from the aromatic ring (nucleophile) attacks the activated electrophile. This creates a new bond and a carbocation. Finally, instead of attacking the carbocation, an additional nucleophile/base removes the original hydrogen, which regenerates aromaticity. The activated electrophile may or may not be cationic. The base may or may not be neutral. These vary depending on the specific reaction, with a generalized reaction depicted.
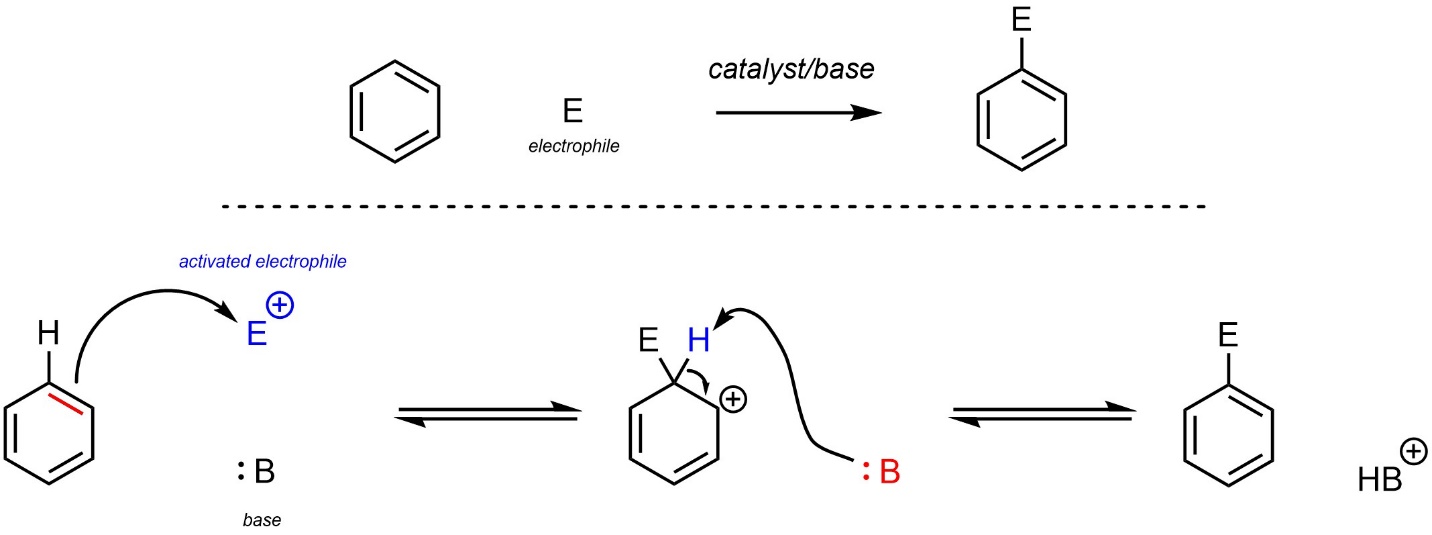
Scheme 10.3 – Generalized Reaction Mechanism for Electrophilic Aromatic Substitution.

