1.6. Lewis Structures
There are a variety of ways to depict chemical structures. The classical approach is through Lewis Structures.
A Lewis Structure displays the atoms of the molecule in the order they are bonded. It also depicts how the atoms are bonded to one another, for example as single, double, and triple covalent bonds. Covalent bonds are shown using lines. The number of lines indicates whether the connection is a single, double, or triple covalent bond. All atom labels are shown and all lone pairs are shown (Figure 1.34).
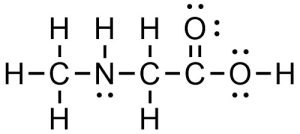
Figure 1.34 – Example of a Lewis Structure.
Notably, Lewis Structures do not attempt to show geometry; all angles are normally shown as 90° and no three-dimensional features are indicated.
1.6.1. Formal Charges
Some atoms carry formal charges (they are cations or anions). This typically happens when the atom has either an incomplete octet or a different number of bonds than usual (see Section 1.2). As such, formal charge may be calculated using the following formula:
Formal Charge = (# of valence electrons) – (# of bonds) – (# of non-bonded electrons)
The formal charge is then indicated by placing a circled positive or negative symbol adjacent to the atom with the formal charge (Figure 1.35).
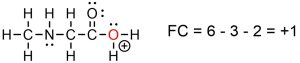
Figure 1.35 – Example of a Formal Charge in a Lewis Structure.
1.6.2. How to Draw Lewis Structures
Drawing Lewis Structures from molecular formulae can be complicated by the fact that there is often more than one structure (molecule) possible. It is much easier to convert so-called condensed formulae, which show the order of atoms like a Lewis Structure but are written in a single line to save space. The order of the atoms suggests the connectivity in the molecule. This provides hints for how to construct the Lewis Structure of the specific molecule, instead of a related compound.
Example: CH3CH2CONH2
- Using the condensed formula as a hint, arrange the atoms.
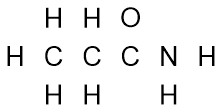
- Add valence electrons around the atoms. If there is a formal charge, add an electron for anionic or remove an electron for cationic.

- Form all obvious σ bonds by combining pairs of electrons from adjacent elements. Follow the Octet Rule.
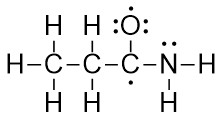
- Form all obvious π bonds by combining pairs of electrons from adjacent elements. Follow the Octet Rule.
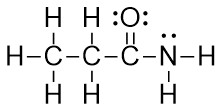
- Confirm that each element in the Lewis Structure follows the Octet Rule. Add formal charges and create additional bonds as needed.

The last step is crucial in ensuring success. Some attempts may generate structures that are not easily resolved. For example, the following arrangement of atoms may have been proposed above:
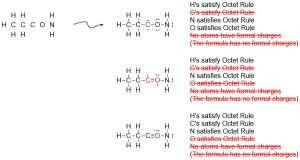
In these cases the structure will never be able to satisfy the Octet Rule for all atoms and contain the proper number of formal charges, so it may be ruled out as a candidate structure.

