7.7. Leaving Groups
Recall that it is important when proposing nucleophile-electrophile reactions to avoid violating the octet rule. For example, if a nucleophile is attacking an electrophile that already has a full octet, the electrophile will have to lose electrons to avoid having more than a full valence. Most often this is done by breaking another bond at the same time the new bond is being formed. In some reactions this may result in a σ bond being broken and another group/atom being ejected from the molecule (see Chapter 11). Atoms or groups of atoms that are ejected as part of the reaction are called leaving groups.
Being able to assess how easily an atom or group can become a leaving group (sometimes called “leaving group ability”) is vital for determining whether certain kinds of reactions are possible. This has applications for proposing reactions but also helps with determining whether a given reaction/step would be reversible or irreversible. For example, nucleophilic attacks from hydrides, Grignard reagents, and organolithium reagents onto ketones are never reversible.
Leaving group ability is inversely proportional to the basicity of the leaving group: stronger bases are poorer leaving groups, weaker bases are better leaving groups.
Generally, the conjugate bases of strong acids (pKa < ~3) are very good leaving groups, the conjugate bases of moderate acids (pKa ~3-6) are good to moderate leaving groups, and the conjugate bases of weaker acids (pKa ~6-12) are moderate to poor leaving groups (Figure 7.16). An exception is fluoride (F–), which is the conjugate base of a strong/moderate acid (HF, pKa = 3.2) but which is a terrible leaving group. The reasons for this exception are beyond the scope of this text.

Figure 7.16 – Examples of Using pKa to Evaluate Leaving Group Ability.
This approach may be used to evaluate whether a proposed step or reaction is feasible. In order for nucleophilic attacks from hydrides or organometallic compounds to be reversible, hydrides or carbanions would have to leave (Scheme 7.18). As these are very strong bases, they are very poor leaving groups, which makes these steps irreversible.
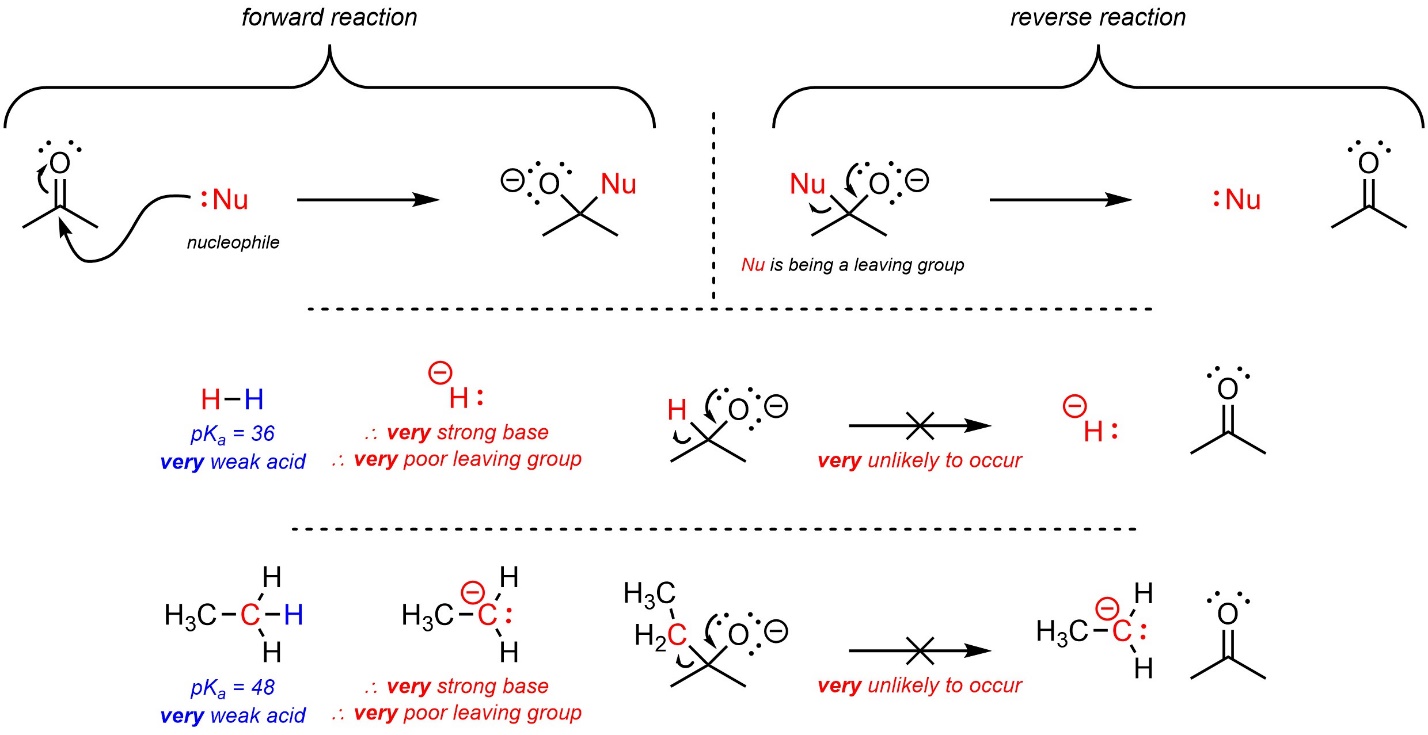
Scheme 7.18 – Examples of Using Leaving Group Ability to Evaluate Reverse Reaction Feasibility.

