8.7. Reaction: Addition of X-X
It is possible to add a halogen (X; X = F, Cl, Br, I) and another halogen (X; X = F, Cl, Br, I) across an alkene (Scheme 8.21). Water and alcohols must be rigorously excluded from the reaction (see Section 8.8.). This is sometimes referred to as halogenation.
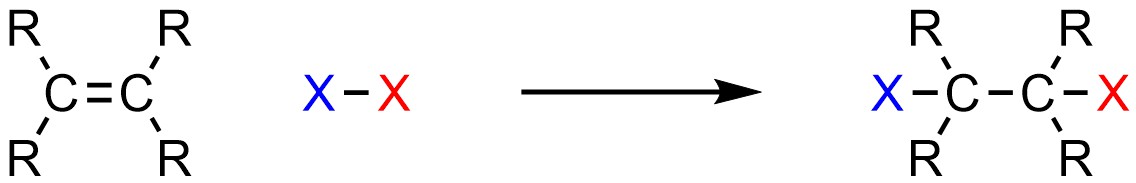
Scheme 8.21 – Generalized Reaction Equation for Addition of X-X Across an Alkene.
8.7.1. Mechanism and Rate-Determining Step
The mechanism for this reaction is often compared to oxymercuration (Scheme 8.22). First, one of the halogens reacts with the alkene to create a three-membered ring. This step is equivalent to the alkene (nucleophile) attacking the halogen (electrophile) at the same time that the halogen (nucleophile) attacks the “carbocation” (electrophile). Halogens are able to do both steps at the same time. Although this avoids the formation of a high-energy carbocation, it instead produces a high-energy halonium (a cationic halogen with two bonds). Halide (nucleophile) then attacks the carbon (electrophile). Although the carbon is not a carbocation, the C-X bond is both weak and polar due to the cationic charge on the halogen. The geometry of the halonium forces a stereospecific addition (see Section 8.7.3.).
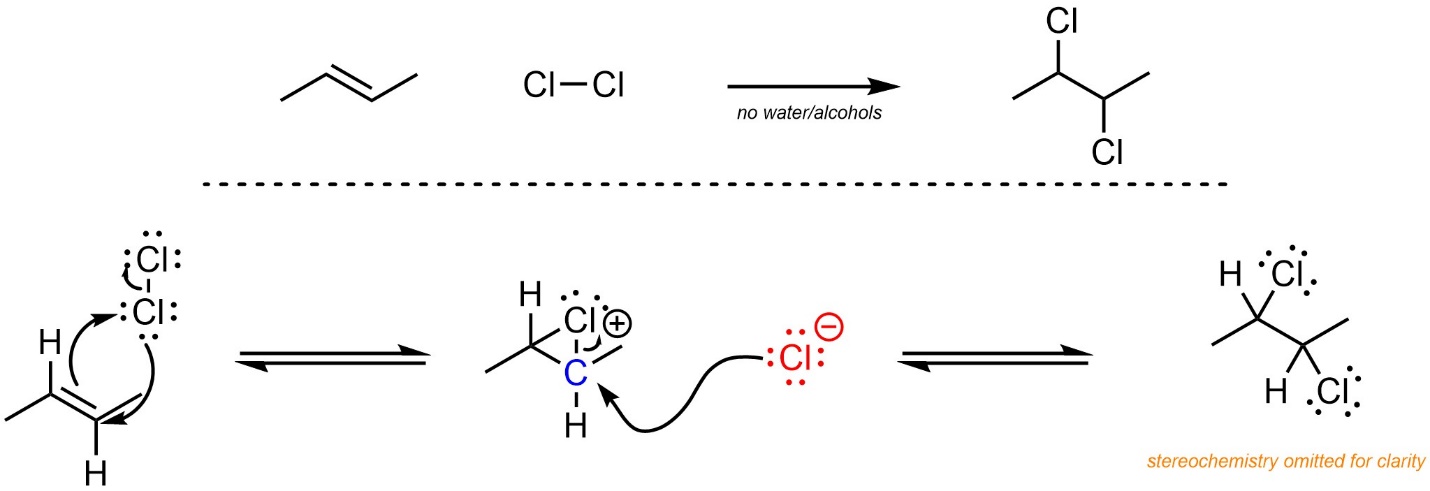
Scheme 8.22 – Reaction Mechanism for Addition of Cl-Cl Across the Alkene of (E)-But-2-ene, Stereochemistry Omitted.
Although no carbocation is formed, a high-energy halonium is only slightly less energetic. The rate-determining step is the formation of the halonium. The overall reaction is exergonic.
8.7.2. Regioselectivity
Because both groups being added are identical, there are no regioisomers and regioselectivity is not a concern. If there is a difference between the two electrophilic positions during the nucleophilic attack of the halide it will prefer to attack the site that would be the more stable carbocation.
8.7.3. Stereoselectivity – Stereospecific: trans
Additions of X-X across alkenes are stereospecific (diastereospecific). The initial step results in the formation of a halonium intermediate. This is a three-membered ring, so both C-X bonds must be above or below the previous plane of the alkene (Scheme 8.23). This geometric constraint imposes an electronic constraint.
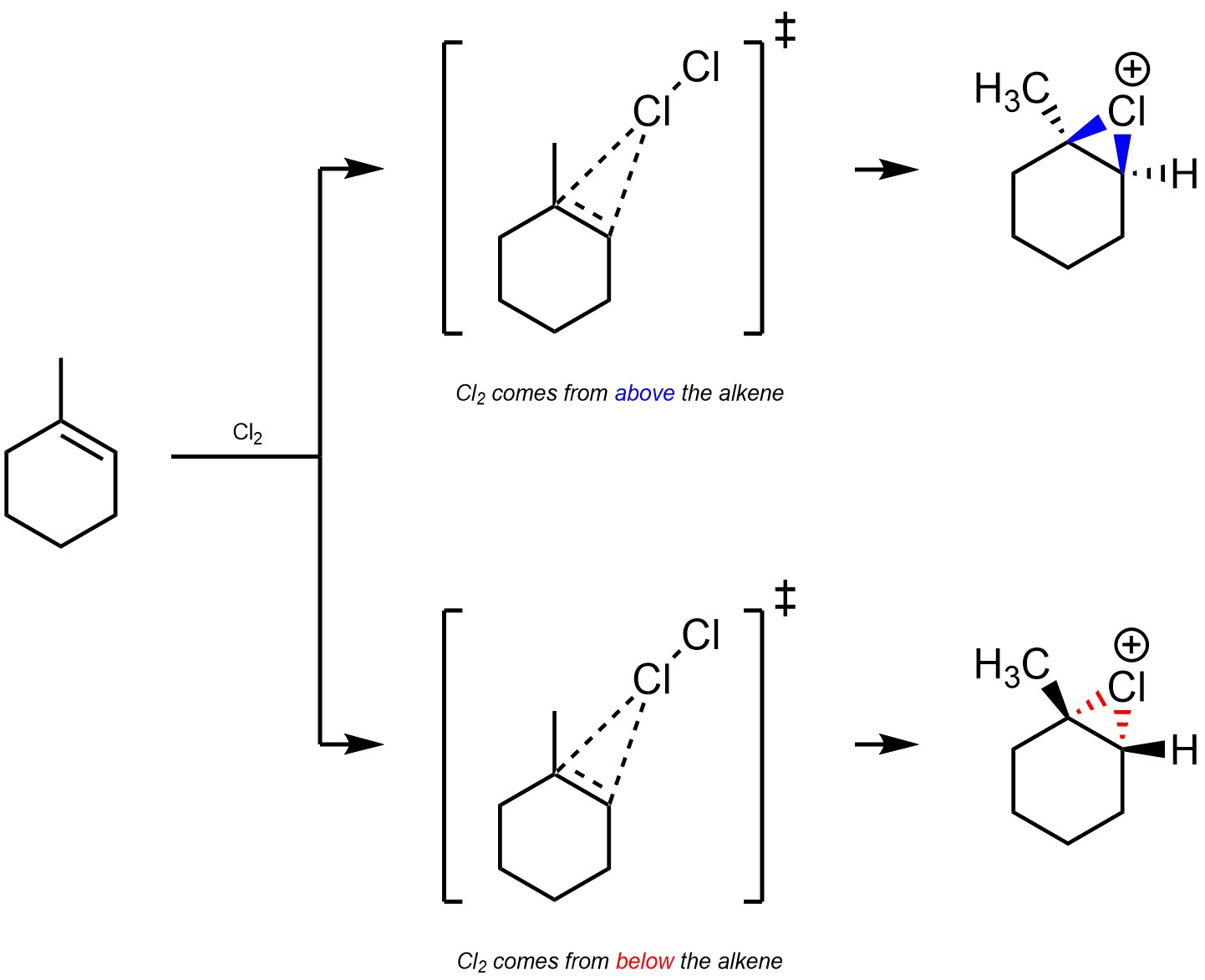
Scheme 8.23 – Formation of Halonium from Chlorine and the Alkene of Methylcyclohexene.
Recall that when two orbitals overlap constructively they form a covalent bond (σ or π; see Section 1.3.1). However, when they overlap deconstructively they form an anti-bonding orbital (σ* or π*; see Section 1.3.1). Usually, in order to break a covalent bond electron density must be put into its corresponding anti-bonding orbital. This also makes a new bond between the atom that donated the electrons and the atom that was attacked. Very often this does not affect acid-base/nucleophile-electrophile reactions because conformational changes make the anti-bonding orbitals freely accessible. However, it is not geometrically possible to orient the corresponding σ* orbitals from the C-X σ bonds so that they face the same direction as the bonds themselves (Figure 8.8).
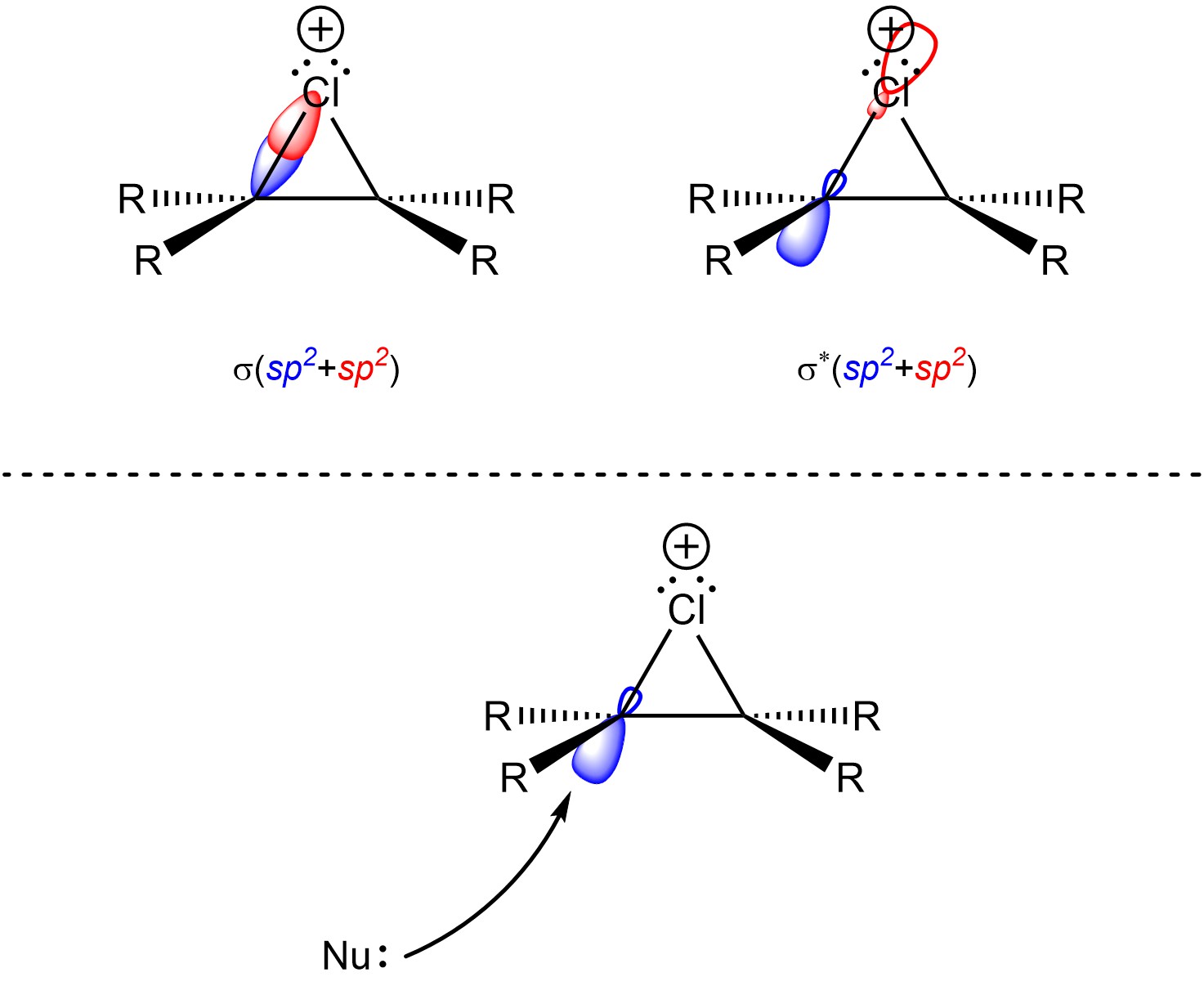
Figure 8.8 – A σ C-Cl Bond and the Corresponding σ* Anti-Bonding Orbital; Representation of the Geometric Constraint of Nucleophilic Attacks into the Corresponding σ* Anti-Bonding Orbital.
The halide could attack the σ* orbital located on the cationic halogen. This is what occurs when the first step is reversed (regenerating the starting materials; see Scheme 8.22). It is only possible for the second halogen to add to the carbon by attacking from the opposite side of the halonium (Scheme 8.24). This is called trans addition, as the two new substituents must be added trans to each other (older sources may refer to this as anti addition).
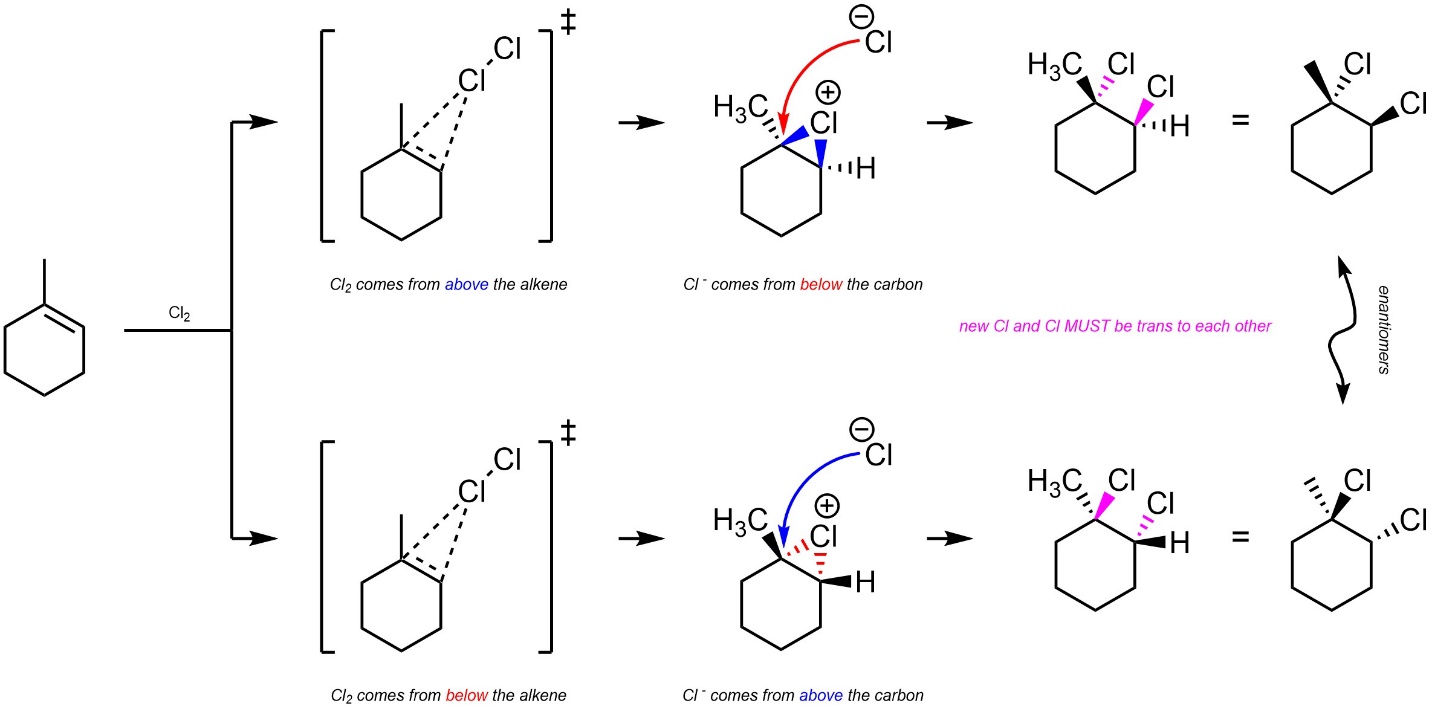
Scheme 8.24 – Diastereospecific Addition of Cl-Cl Across the Alkene of Methylcyclohexene.
It is very important to remember that this is a form of diastereoselectivity (by being diastereospecific). There is no enantioselectivity.

