10.9. Reaction: Acylation via Friedel-Crafts
It is possible to change a hydrogen (H) into an acyl group on an aromatic ring (Scheme 10.14). Acyl groups are related to alkyl groups but have a carbonyl on the carbon attaching them. This reaction was specifically developed to overcome the limitations of Friedel-Crafts Alkylation (see Section 10.8.3.) and is often referred to as Friedel-Crafts Acylation.
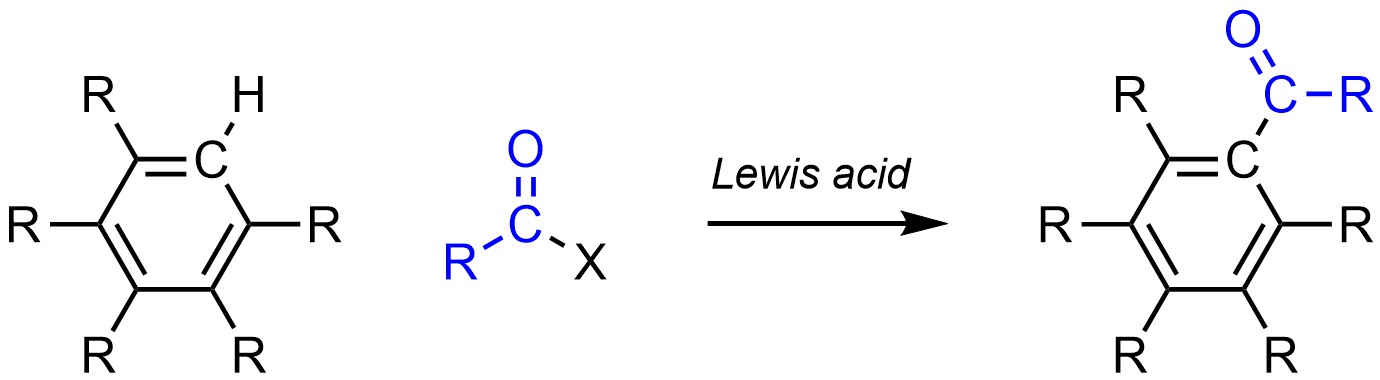
Scheme 10.14 – Generalized Reaction Equation for Friedel-Crafts Acylation of an Aromatic Ring.
10.9.1. Catalyst Requirements
Directly using an acid halide (sometimes called acyl halide) will not result in acylation without a catalyst. The catalysts used are Lewis acids, and work by coordination with the halide. Although many Lewis acids are able to catalyze these reactions (see Table 10.1), FeBr3 and AlCl3 are the most commonly used. This is due to convenience rather than any catalytic benefit over other Lewis acids.
10.9.2. Mechanism
The overall mechanism for this reaction has the same two main steps but has an additional activation step for the catalyst (Scheme 10.15). The acid halide (nucleophile) reacts with the Lewis acid catalyst (electrophile) to form an ionic complex. Because of resonance stabilization from the carbonyl, the complex ejects MX4– and forms a stabilized carbocation (an acylium ion). This greatly increases its electrophilicity. Then, a π bond from the aromatic ring (nucleophile) attacks the carbon (activated electrophile). This creates a new C-C bond and a carbocation. Finally, the halide (nucleophile/base) removes the original hydrogen (electrophile/acid), which regenerates aromaticity and the catalyst. Although halides are very weak bases, the last step is favourable because it regenerates aromaticity.
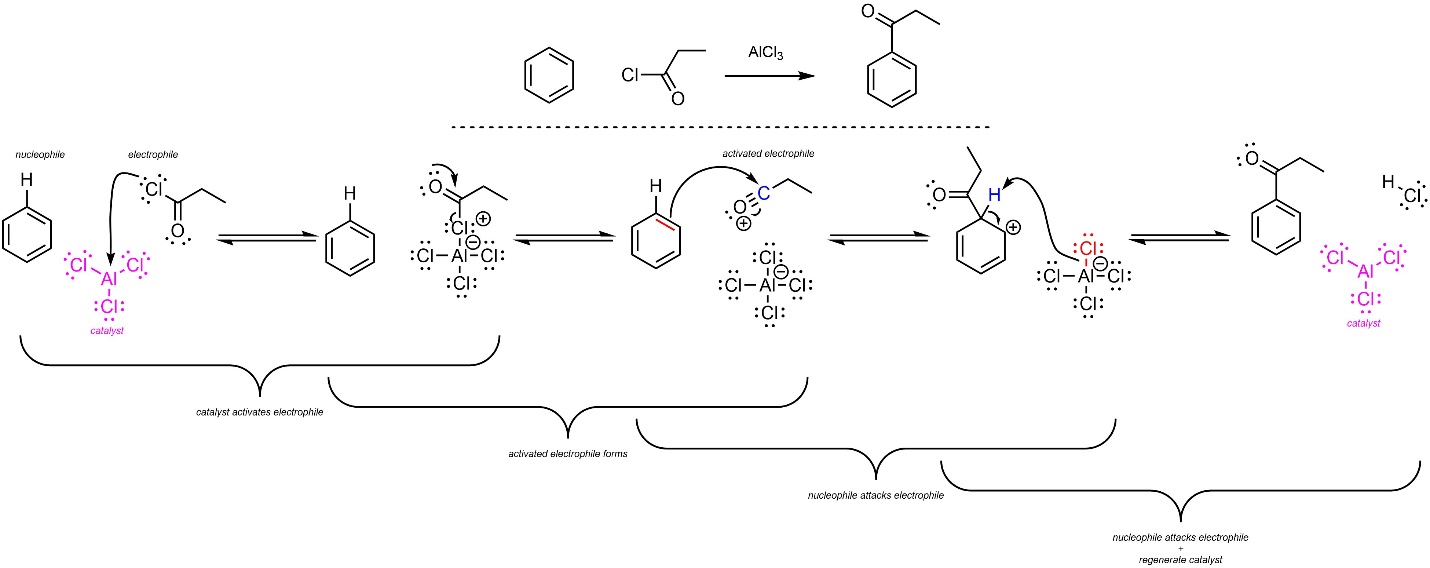
Scheme 10.15 – Reaction Mechanism for Friedel-Crafts Acylation of Benzene Using Propionyl Chloride and Aluminum Trichloride.
10.9.3. Contrast with Alkylation via Friedel-Crafts
Like its alkylation counterpart, Friedel-Crafts acylation will not work well if the aromatic ring possesses any functional group that has one or more lone pairs (see Figure 10.2). Functional groups with lone pairs will react with the Lewis acid catalyst.
Because of the differences between acyl and alkyl groups (see Sections 10.10.1. and 10.10.2.) the product is less reactive than the starting material. As a result, it does not undergo additional acylation(s) (Scheme 10.16).
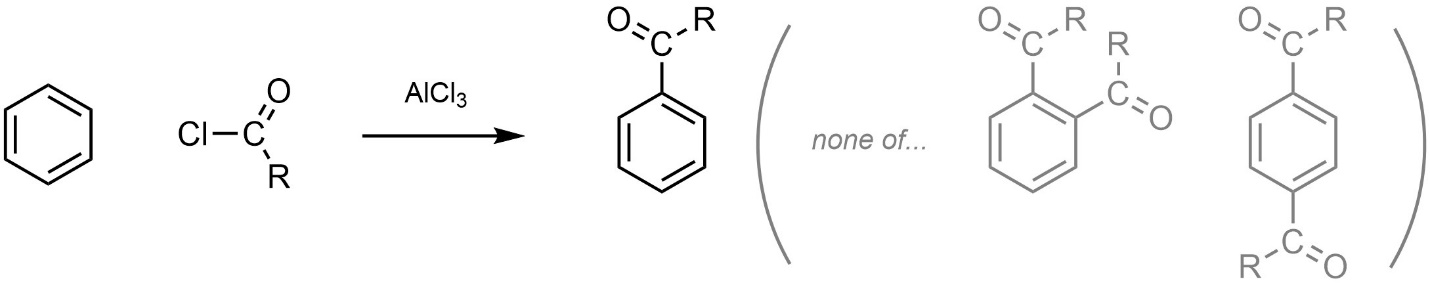
Scheme 10.16 – Highlighted Lack of Multiple Acylation Side Products in Friedel-Crafts Acylation.
Because the carbocation (acylium ion) is resonance stabilized (Figure 10.3) it does not readily undergo other reactions instead of (or in addition to) the desired reaction.
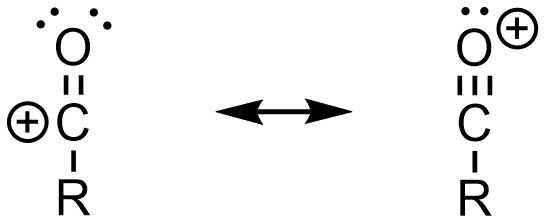
Figure 10.3 – Resonance Stabilization of Acylium Ions.
Combined, these factors often result in higher yield of the product for Friedel-Crafts acylation reactions than for Friedel-Crafts alkylation reactions

