4.4. Stereoisomerism From Pi (π) Bonds
Technically, under the definition of stereogenic centres (see Section 4.2.2.3.) the carbon atoms in most alkenes are stereocentres. Each is a grouping of atoms consisting of a central atom (each of the carbon atoms in the alkene) and distinguishable substituents, and the interchange of two of the substituents leads to a stereoisomer (Figure 4.31). However, most chemists do not refer to these as stereocentres because they are not independent of each other: changing the arrangement of substituents around one of the carbon atoms is equivalent to having changed the substituents around the other carbon atom. As a result, in this text we will not consider carbon atoms in alkenes as stereocentres. This type of stereoisomer falls under the broader category of stereogenic elements, which is not covered in this text.
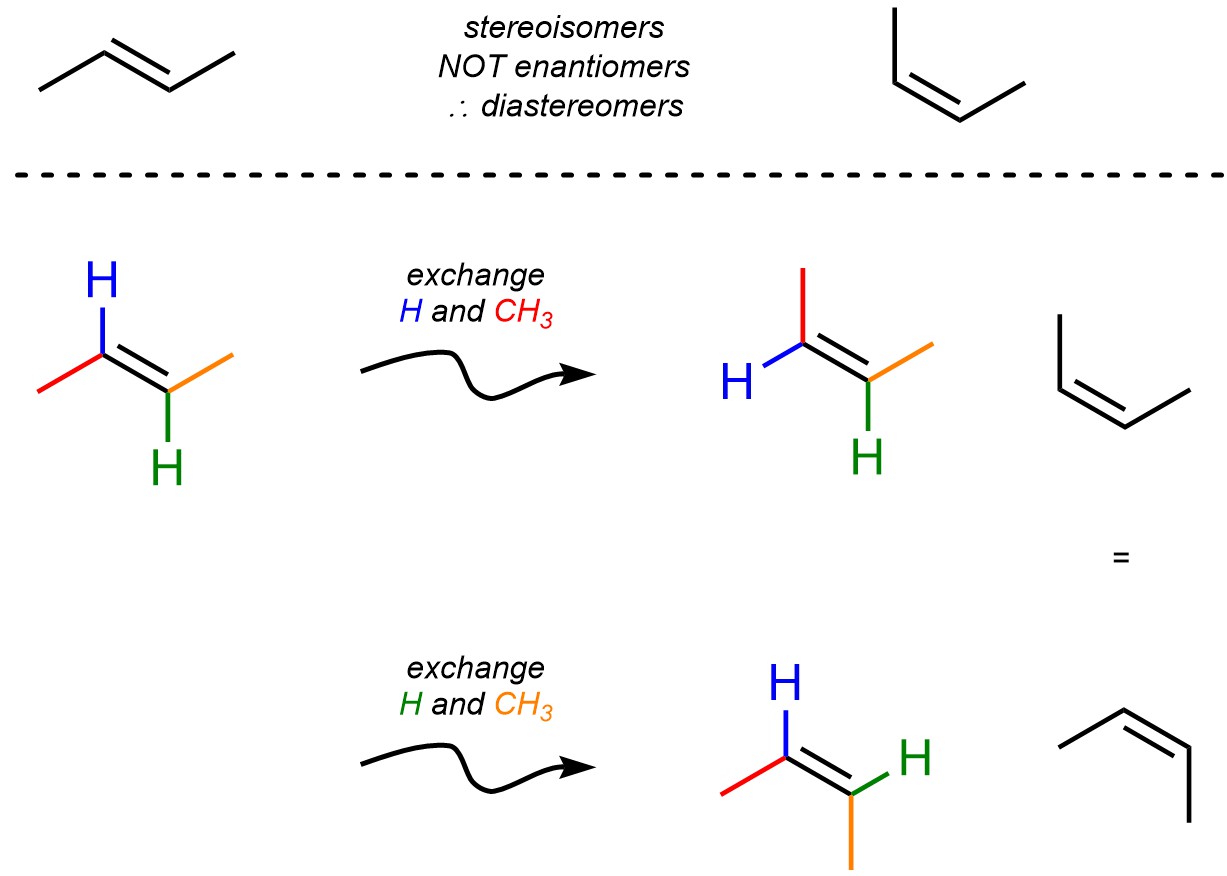
Figure 4.31 – Stereoisomerism Around Alkenes.
Recall that molecules that have the same chemical formula AND their atoms are connected in the same sequence BUT they differ in the three-dimensional arrangement of those atoms are called stereoisomers. Since all stereoisomers that are not enantiomers are classed as diastereomers, and the two stereoisomers above (Figure 4.31) are not non-superposable mirror images of each other, they must be diastereomers.
For some students this generates confusion when assessing the relationship between compounds (Figure 4.32). The simplest way to counter this is to always keep the definition of enantiomer in mind; since alkenes are planar (both carbon atoms are sp2 hybridized) the geometry around them will not change when reflected through a mirror.
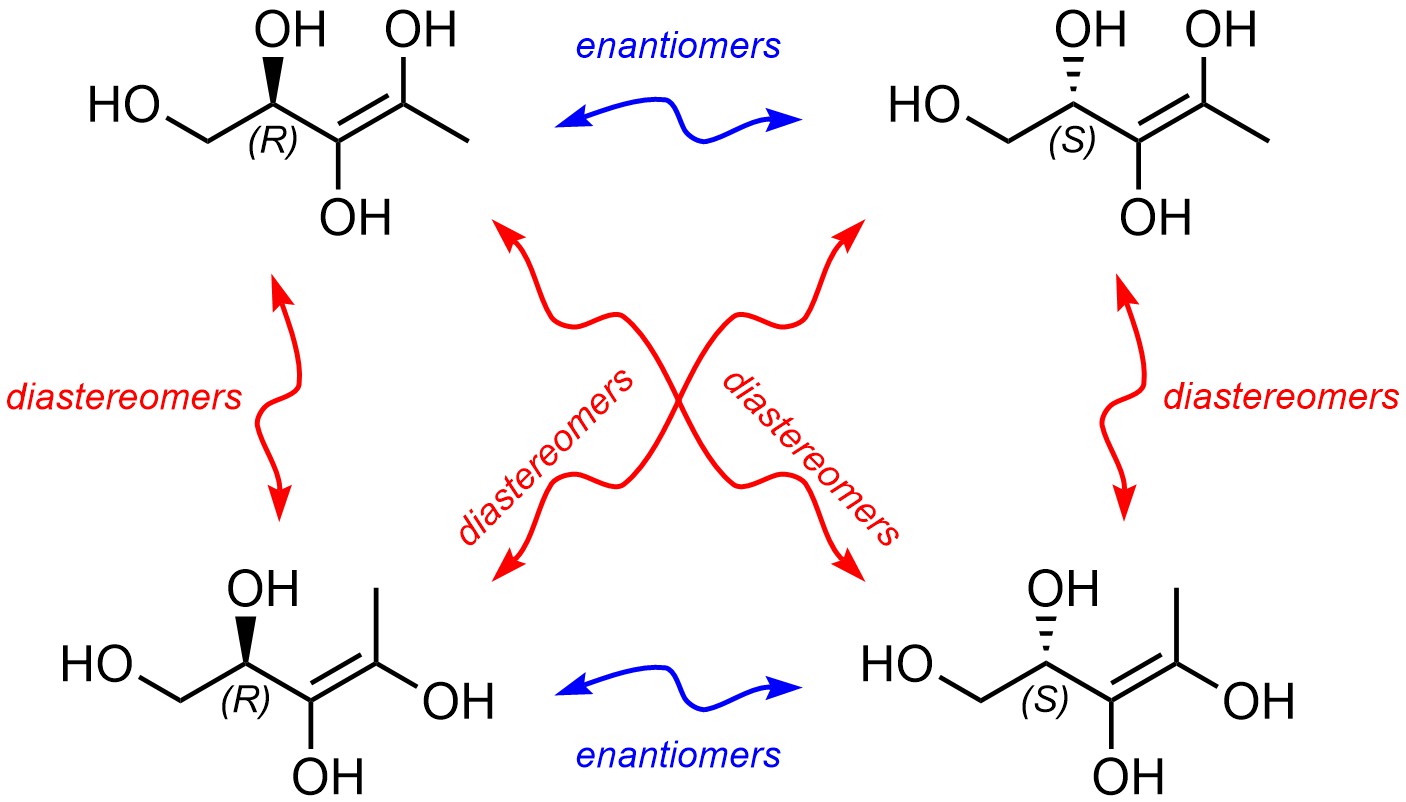
Figure 4.32 – Stereoisomers of Pent-3-ene-1,2,3,4-tetraol Highlighting Relationships.
Other types of π bonds may exhibit stereoisomerism. For example, imines and iminium ions can exists as stereoisomers (Figure 4.33). These types of compounds are not common in this text, but it is important to recognize that they are possible.
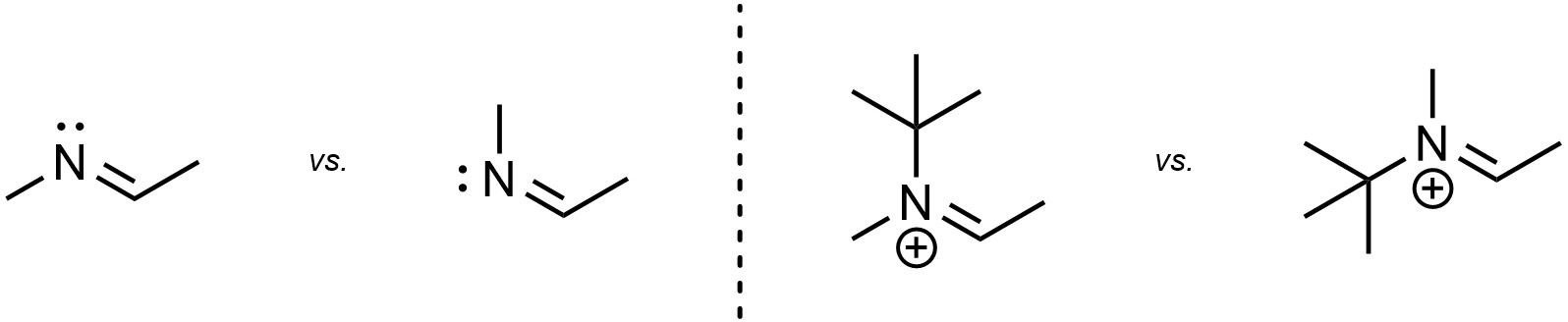
Figure 4.33 – Examples of Stereoisomerism from Other π Bonds.
4.4.1. CIP Nomenclature and Configuration
As with stereocentres, the configuration of alkenes can be determined using the Cahn-Ingold-Prelog (CIP) system. Instead of R or S, alkenes are classified as E or Z. For example, the two stereoisomers of but-2-ene are properly called (E)-but-2-ene and (Z)-but-2-ene (Figure 4.34).
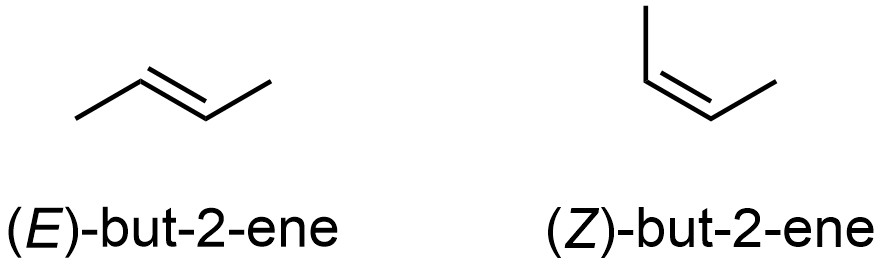
Figure 4.34 – Configuration of the Stereoisomers of But-2-ene.
The rules for differentiating these can be broadly summarized as assigning priority to the substituents (based on atomic number) and determining whether the high priority substituents are on the same or opposite sides of the alkene.
4.4.2. How to Assign E/Z Configurations
The systematic way of assigning configuration for alkenes is simpler than for stereocentres. With practice the process can usually be streamlined, and a configuration assigned nearly instantaneously.
Determine which alkenes can exhibit stereoisomerism. If exchanging the two groups on one end does not generate a stereoisomer then assigning E and Z to that alkene is not possible. Having two identical substituents is the most common reason for this.
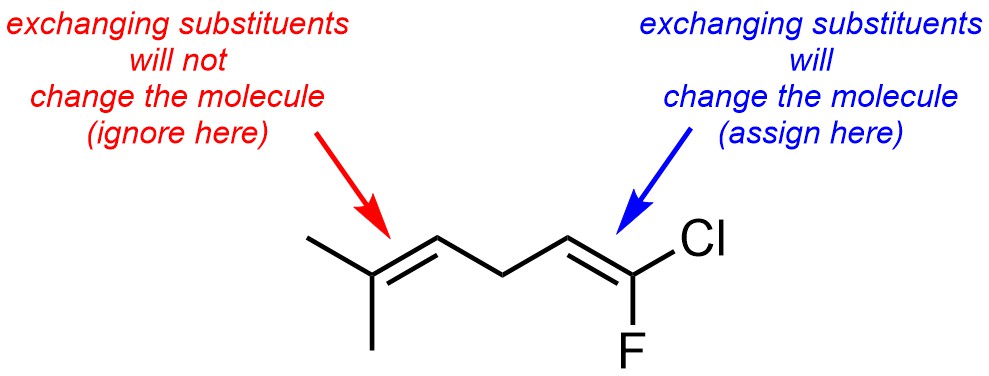
Assign priority to each group. Priority is based on atomic number. The highest priority group is assigned as 1 and the other is 2. Do this to both carbon atoms of the alkene. As with stereocentres, if there is a tie move down the chain and compare the atoms attached to each side.
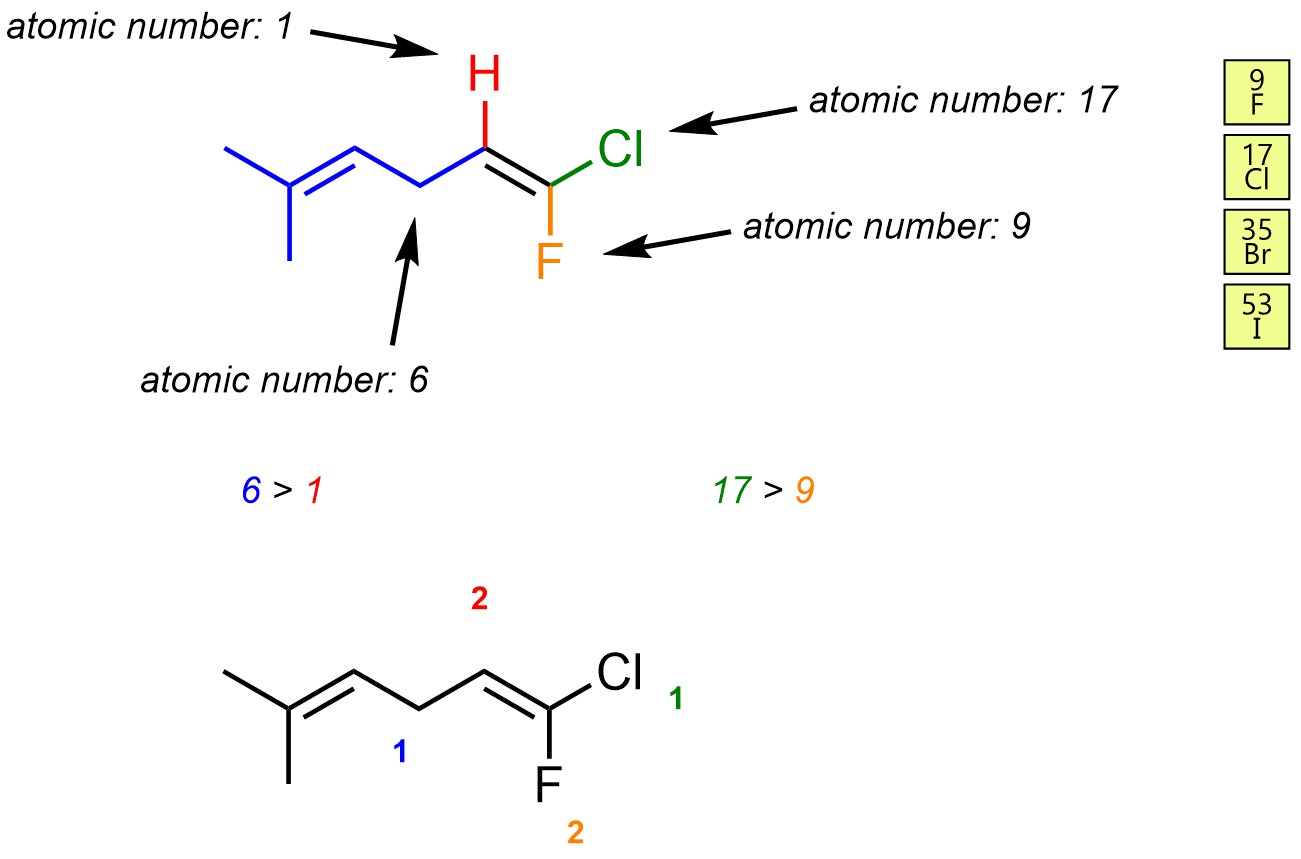
If the two high priority groups are on the same side of the alkene, the absolute configuration is Z. If the two high priority groups are on opposite sides of the alkene, the absolute configuration is E.
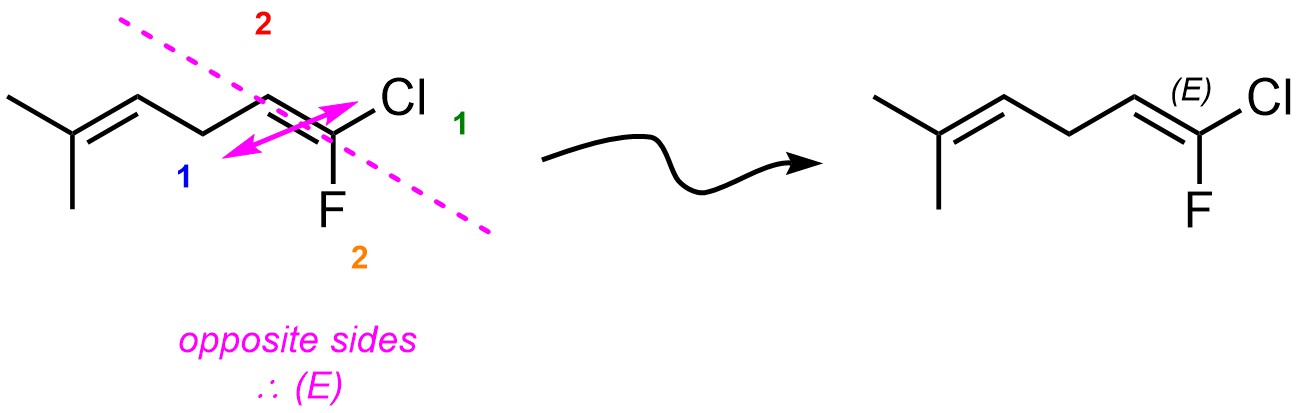
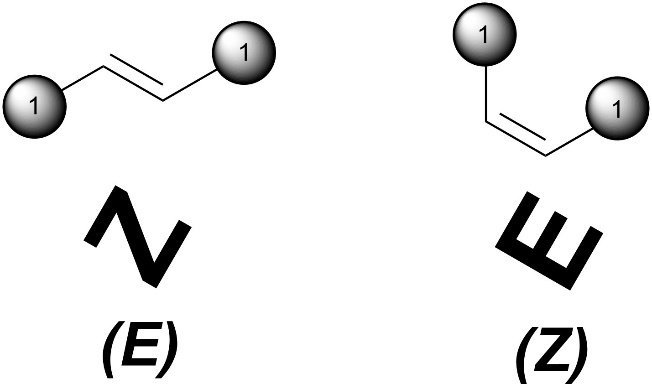
There is a mnemonic to help assign absolute configurations to alkenes. Look at the shape made from only the high priority groups and the alkene. If it looks like a Z, it’s (E) and if it looks like an E it’s (Z). Creativity may be needed to see the E shape.
Some older sources classify alkenes using a cis and trans system. This approach is only valid for some alkenes, generally simple ones. At its core, the cis/trans system is limited because these are relative descriptors. The term “cis” means that two things are on the same side of something relative to each other. The term “trans” means that two things are on opposite sides of something relative to each other. For example, the molecule below can be described using phrases like “the fluorine is cis to the methyl group”, “the fluorine is trans to the hydrogen”, “the chlorine is trans to the methyl group”, or “the chlorine is cis to the hydrogen” (Figure 4.35). However, it cannot be classified overall as a cis or trans alkene. Only the E/Z system can be used to describe its configuration.
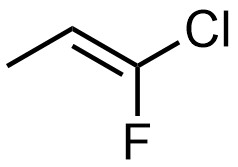
Figure 4.35 – Representation of (E)-1-Chloro-1-fluoroprop-1-ene.
Additionally, these terms may be used to describe other relative relationships. For example, the two diastereomers of 1,2-dimethylcyclohexane may be classified as cis and trans (Figure 4.36). This refers to the relative orientation of the two methyl groups being on the same or opposite sides of the ring with respect to each other. Note that this ignores some aspects of stereochemistry since both enantiomers of the chiral stereoisomer are classified using the same descriptor. This adds yet more ambiguity to the system.
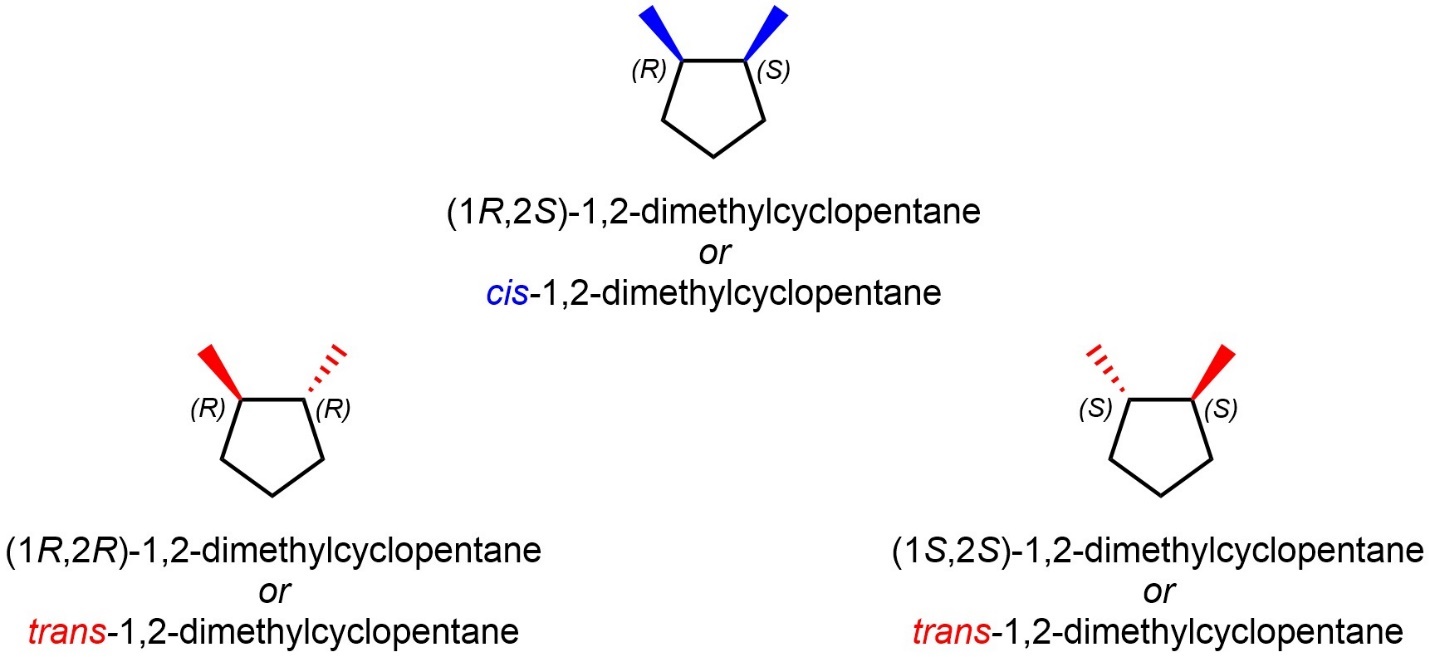
Figure 4.36 – Absolute Configuration of the Stereoisomers of But-2-ene.
Given the ambiguity and limitations of the cis/trans system its use at an introductory level is discouraged. However, since many sources use these descriptors it is important to understand their meaning.

