3.1. Rotations Around Single (σ) Bonds
All sigma (σ) bonds possess an interesting symmetry: they are cylindrical (see Figures 1.9, 1.10, and 1.11). While this makes for nice images, more importantly it means that either of the orbitals making a σ bond can be rotated arbitrarily along the axis of the bond and it will have no effect on the bond itself. At room temperature molecules (and the atoms that make them) move constantly. Because the two sides of a σ bond can rotate independently without affecting the bond, these kinds of movements are common (Figure 3.1). Barring outside factors (π bonds, rings, geometric constraints) all σ bonds will be capable of rotations like this.
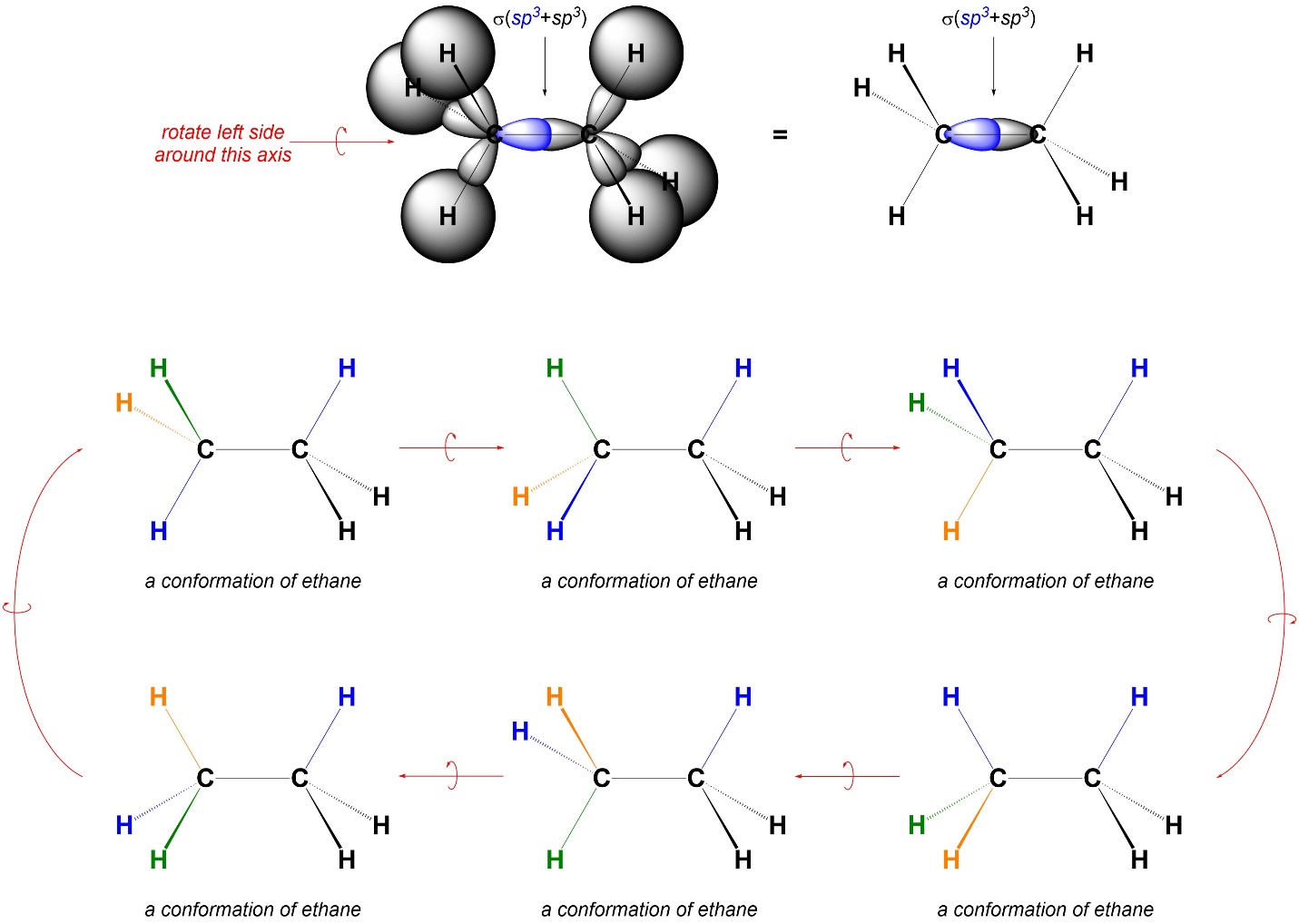
Figure 3.1 – Example of 60° Rotations Around a σ Bond and Resulting Conformations.
Different rotational arrangements are called conformations. Most molecules have infinitely many conformations through rotations along σ bonds; technically every change, even by a fraction of a degree, along a bond counts as a different conformation. However, only some conformations are normally relevant for discussion.
When a conformation is a local energy minimum it is called a conformer. As a result, all conformers are conformations, but not all conformations are conformers. At an introductory level the distinction in terms is not normally important (always using the term “conformation” avoids the possibility of being incorrect). However, being aware of the difference and recognizing both terms is helpful given that most sources will use both.

