Chapter 6 Practice Problems
Answers for these practice problems are on the next page.
A good approach is to answer all of the questions on a piece of paper and then check your answers. This avoids accidentally seeing the answer(s) for questions you have not done yet.
Because Chapter 6 has two approaches for comparing acidities, qualitative and quantitative, most questions will suggest you do them twice (once with each method). This is to familiarize students with qualitative methods rather than just “looking the answer up in a table”. There are two benefits: students may not always have relevant pKa values for comparisons and qualitative approaches are (usually) faster.
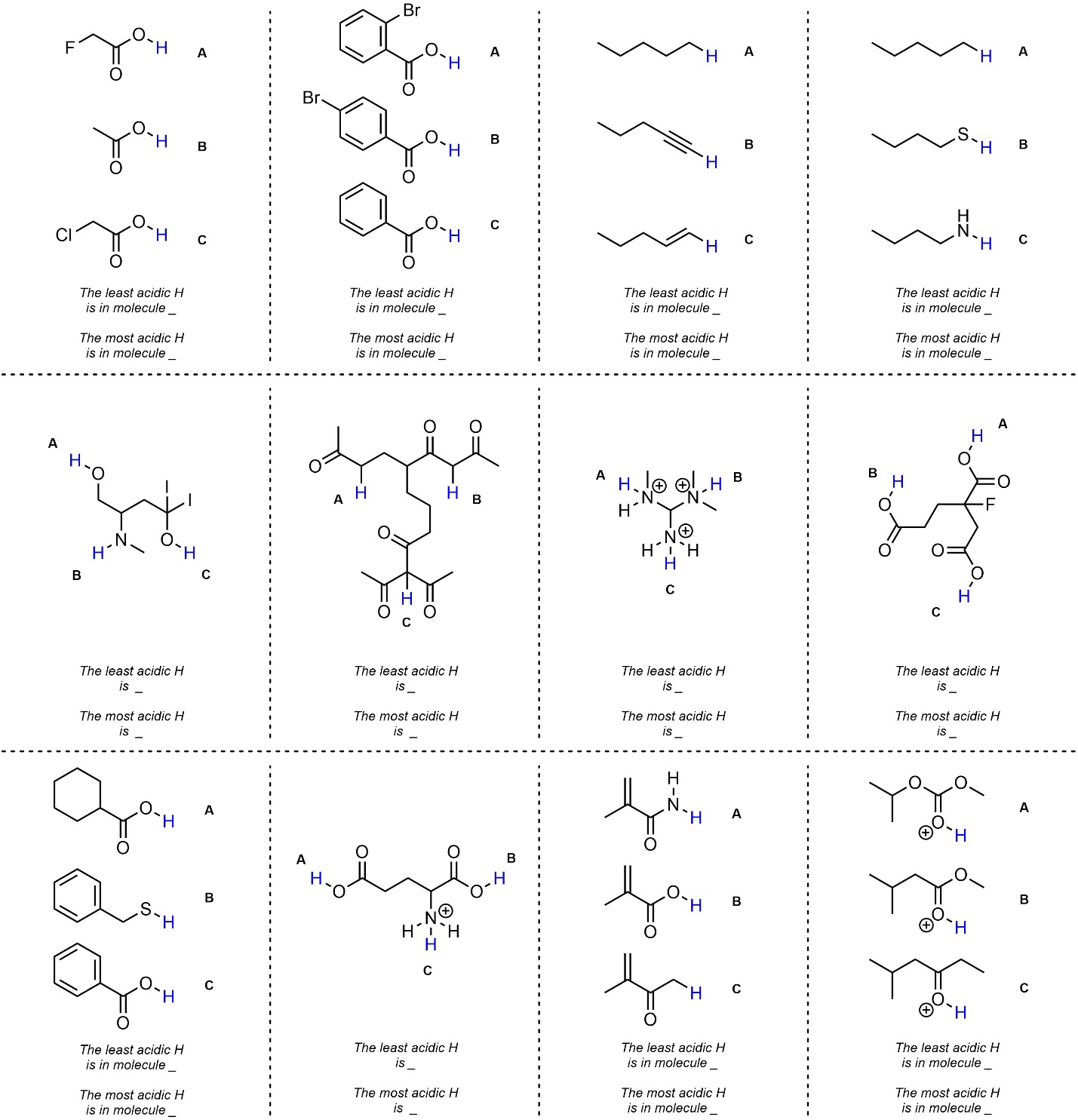
Q6.1: Determine the most and least acidic H’s in each set. The H’s you should compare are highlighted. For practice purposes you should do this twice: first without looking at any pKa values (qualitatively), then again using values from the pKa tables provided in the textbook (quantitatively).
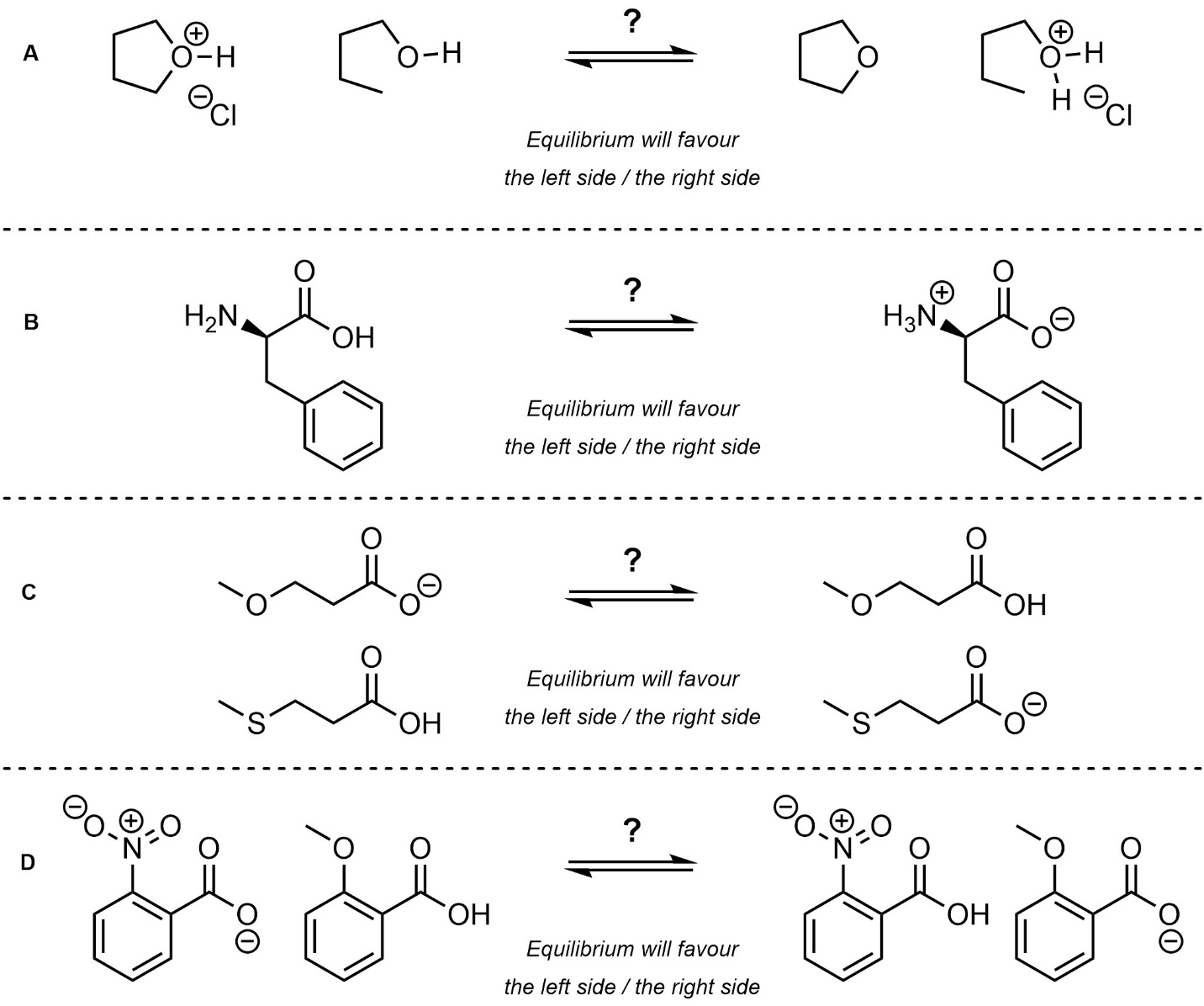
Q6.2: Determine which side of the equilibrium is favoured. For practice purposes you should do this twice: first without looking at any pKa values (qualitatively), then again using values from the pKa tables provided in the textbook (quantitatively).
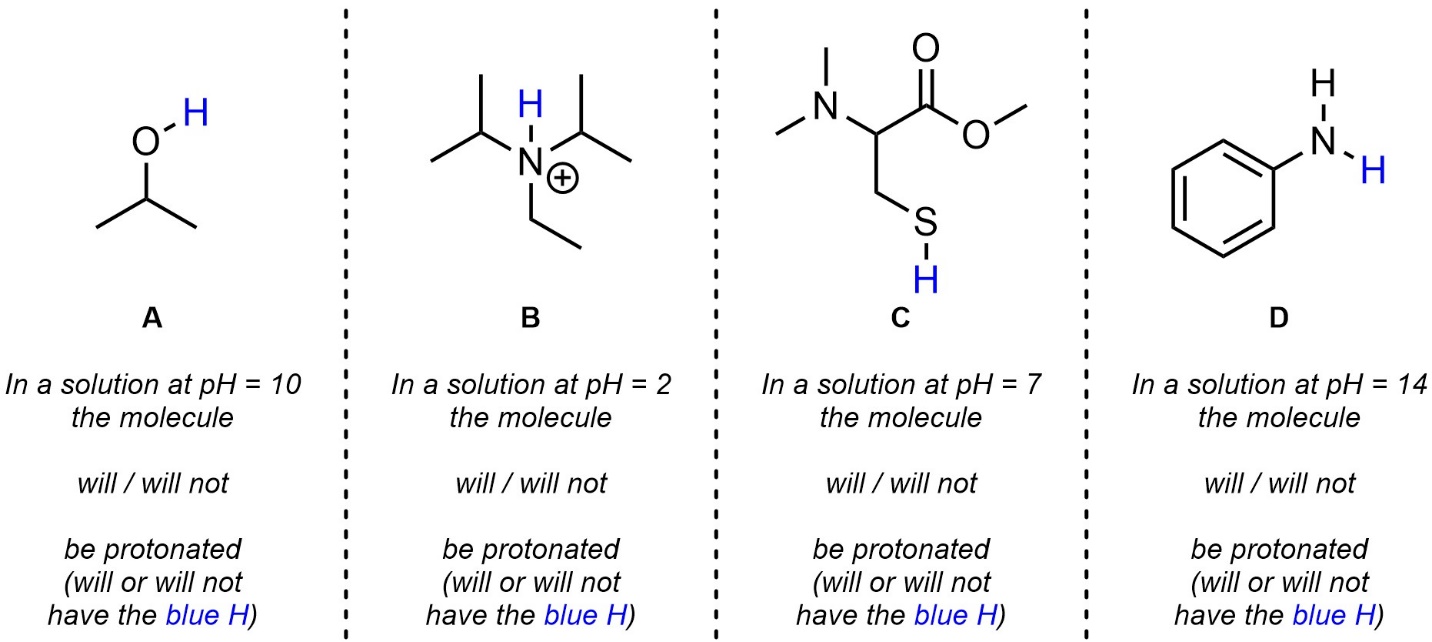
Q6.3: Determine the protonation state of the molecule given the pH of the solution it is in (i.e. whether the molecule primarily exists as shown or as the conjugate base). The H’s you should consider are highlighted. For practice purposes you should attempt this twice: first without looking at any pKa values (qualitatively), then again using values from the pKa tables provided in the textbook (quantitatively).
Note: Because you are comparing to a specific value doing this qualitatively is more challenging; use what you learned/saw from answering the previous questions (qualitatively and quantitatively) to make an informed qualitative choice. Do not be discouraged if your qualitative choice is incorrect as long as you attempted it to the best of your ability.
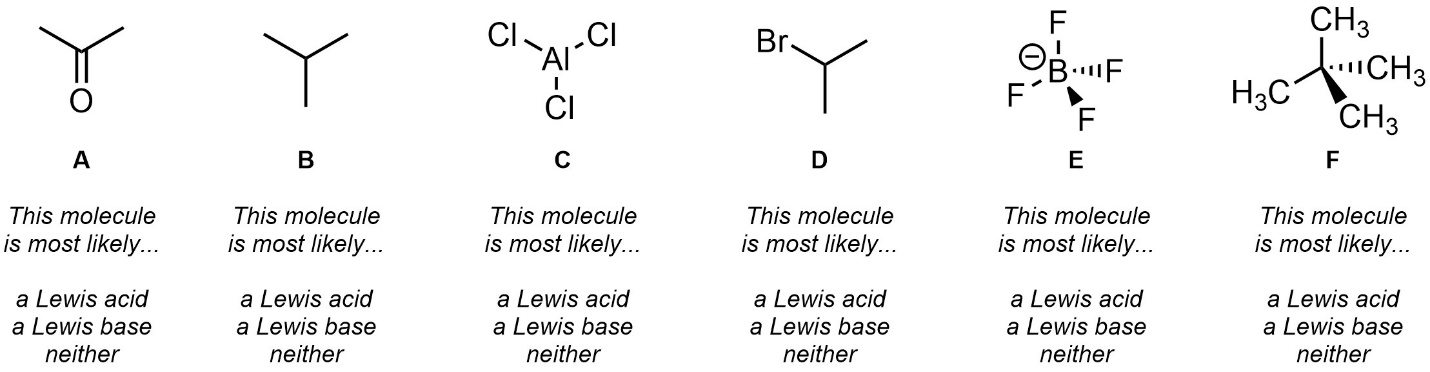
Q6.4: Strictly from memory (without looking up the definitions, searching online, etc.) give a very brief definition of Lewis acid and Lewis base and then class each of the following molecules.