Chapter 5 Practice Problems
Answers for these practice problems are on the next page.
A good approach is to answer all of the questions on a piece of paper and then check your answers. This avoids accidentally seeing the answer(s) for questions you have not done yet.
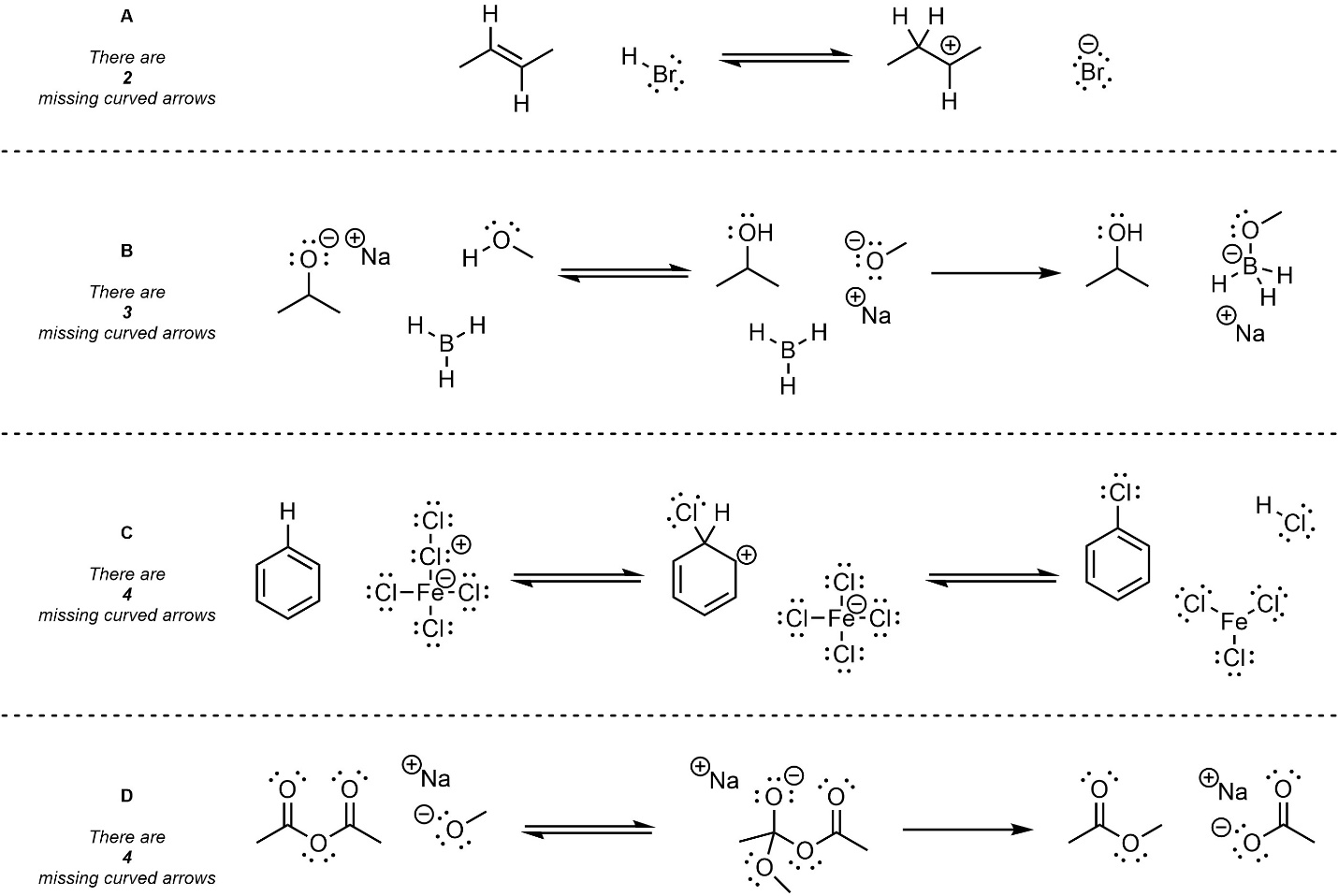
Q5.1: Below are several steps from mechanisms of hypothetical chemical reactions. The only parts missing are curved arrows representing electron flow. Add the missing curved arrows. For convenience the number of missing arrows from each example is given.
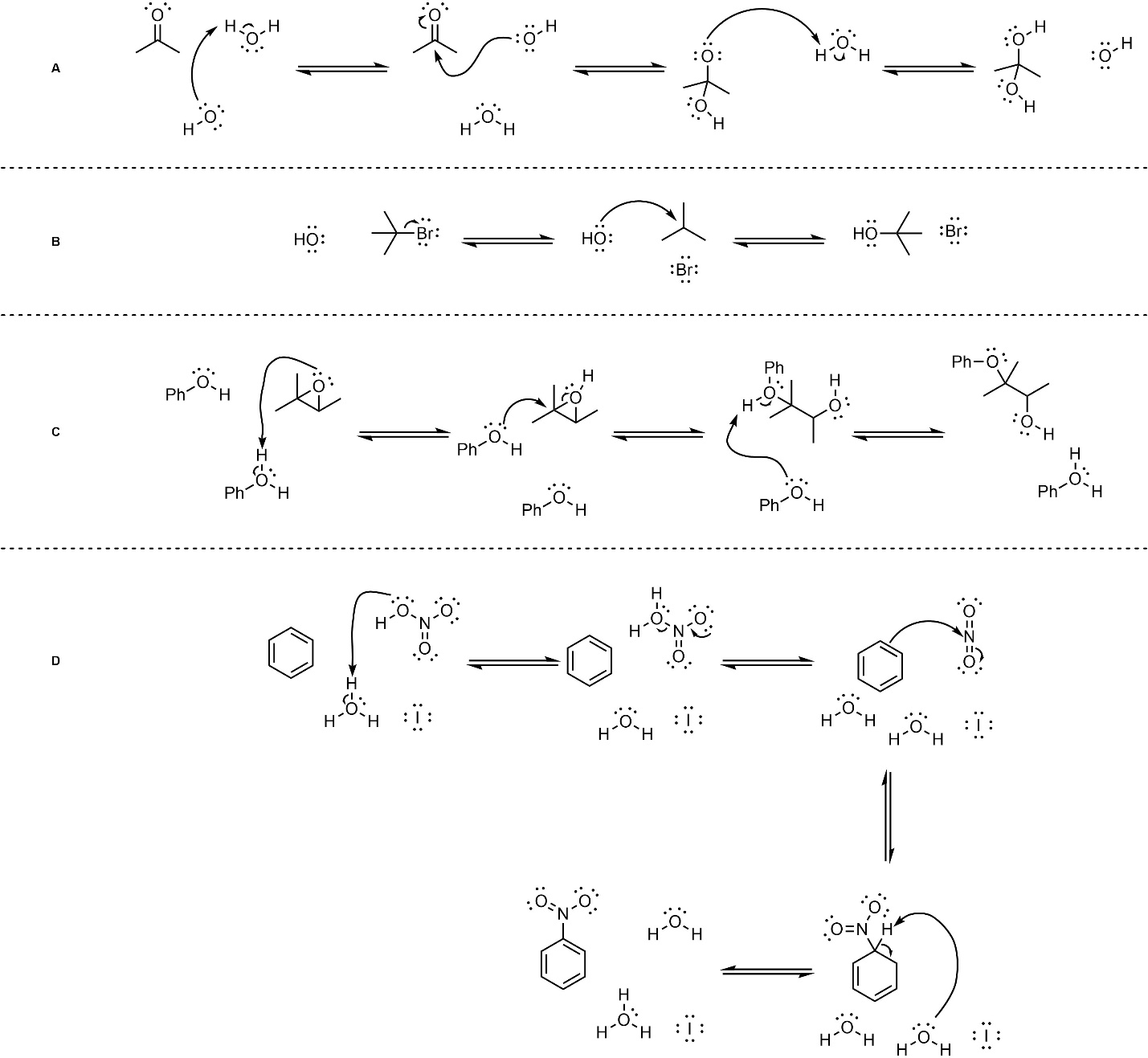
Q5.2: Below are mechanisms of hypothetical chemical reactions. The only parts missing are formal charges. Add the missing formal charges. Some questions are also missing a counterion (i.e. they will not be overall neutral at each step). Do not worry about the missing counterions, they will not affect the mechanism.
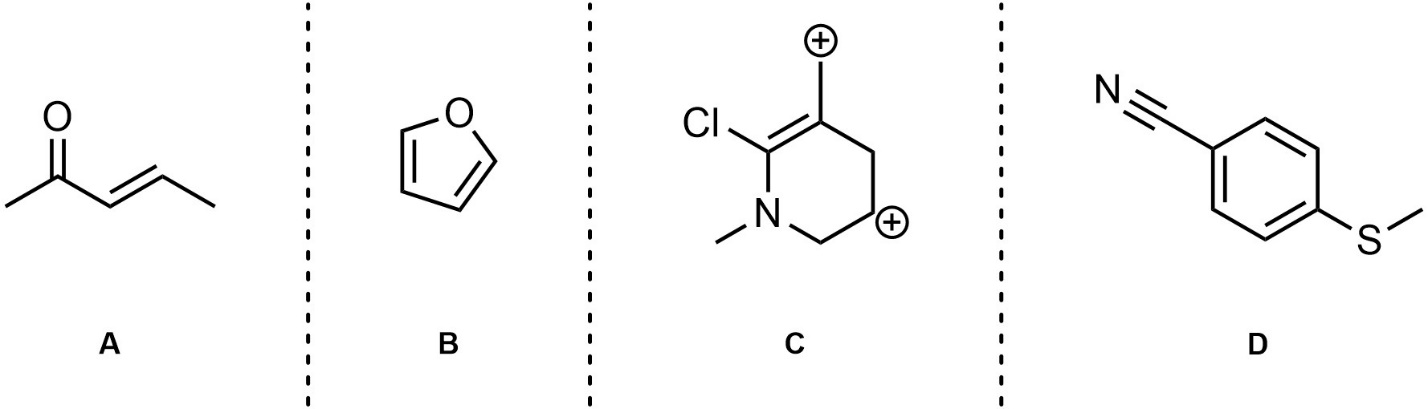
Q5.3: For each of the following draw two additional significant resonance structures. Although it is a significant contributor, for D simply rotating the three pi bonds around the ring does not count (too easy).
Note: Adding all lone pairs and/or showing curved arrows is not required but will greatly assist in answering the question.
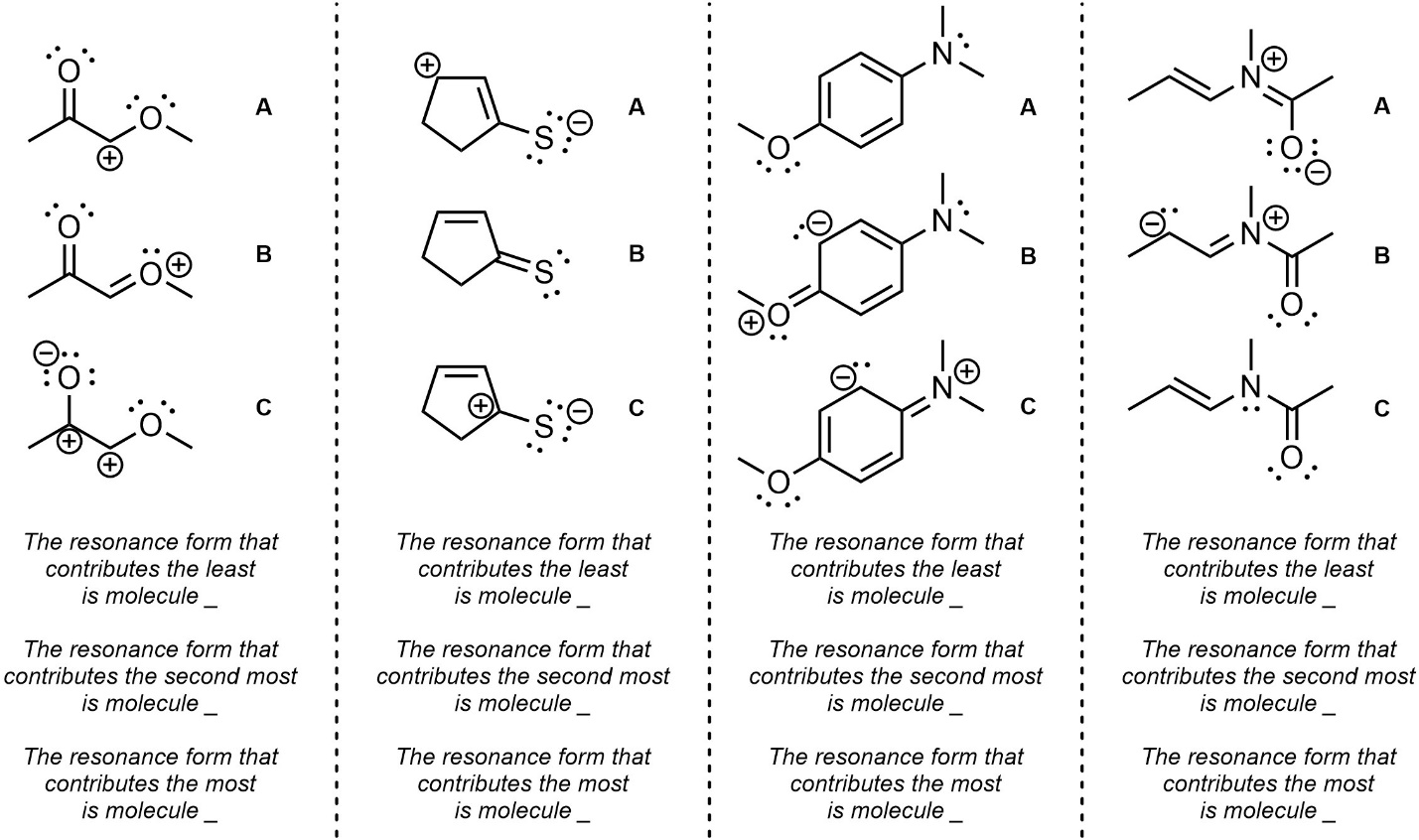
Q5.4: Rank each set of resonance forms in order of increasing contribution (lowest to highest).
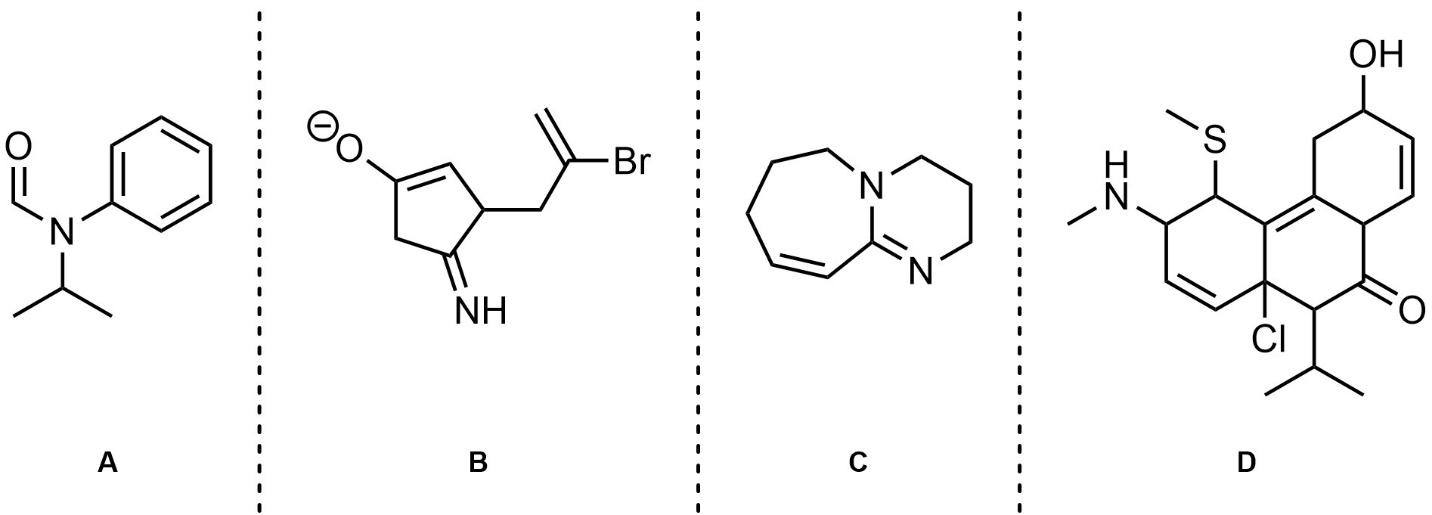
Q5.5: In the resonance forms provided all carbon atoms are neutral. However, in other significant resonance forms some are charged (cationic/anionic). Indicate (circle, draw an arrow to, etc.) which carbon atoms are cationic/anionic in other resonance forms. Differentiate (colour, label, etc.) whether they are cationic or anionic in the other forms.
Note: Drawing additional resonance forms is not required but may assist in answering the question.
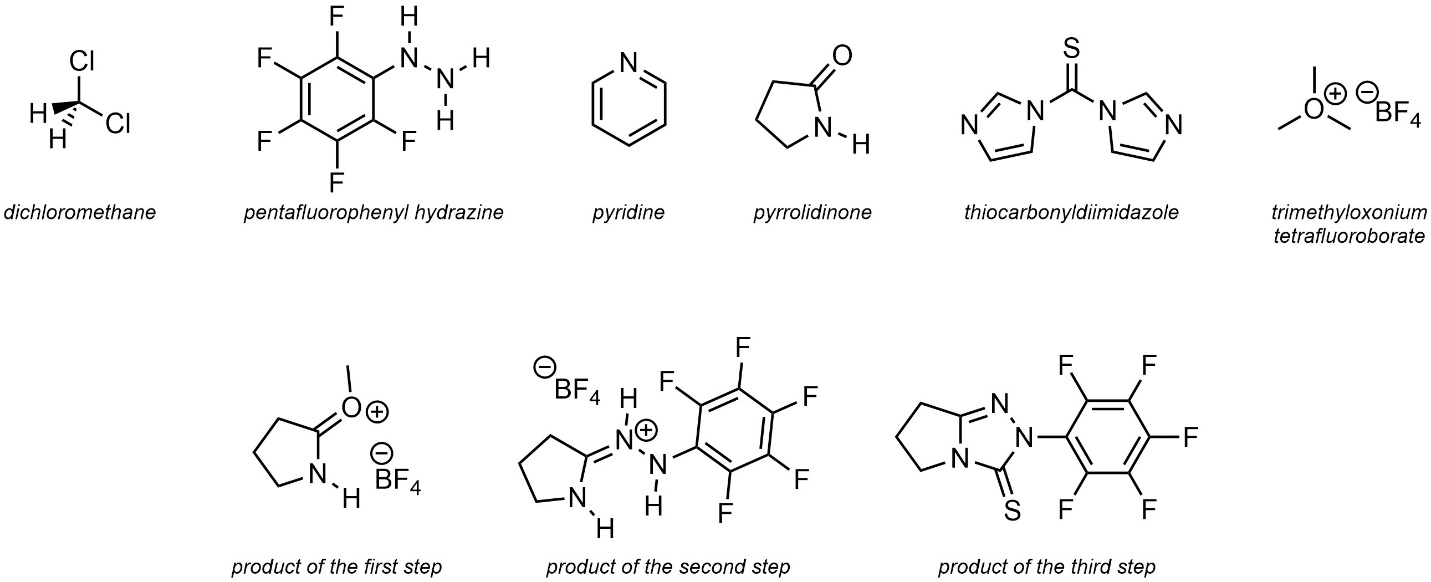
Q5.6: The text below is a procedure for a hypothetical multi-step reaction. Condense the information into a (relatively) simple reaction scheme using “over-the-arrow” notation. For the purposes of practice include all commonly shown species and conditions (e.g. Figure 5.4). It is recommended to use discrete steps, but you may use implied (numbered) steps if you choose. The structures of named compounds and intermediates are included but not all are required for the reaction scheme.
Note: The exact reactions occurring are not important. These compounds are intentionally chosen to be challenging, using names and structures not familiar to the reader to encourage careful consideration of the role of each compound.
Pyrrolidinone (1.00 equiv.) was added to a round bottom flask. The vessel was fitted with a septum, purged under vacuum three times, and placed under inert (argon) atmosphere. Dichloromethane (CH2Cl2 (0.2 M)) and trimethyloxonium tetrafluoroborate (1.00 equiv.) was added. The mixture was then stirred for 24 h at room temperature (23 °C) under the argon atmosphere. Pentafluorophenyl hydrazine (1.00 equiv.) was added in one portion and stirring continued for 48 h at 0 °C. The solvent was evaporated. The resulting oil was suspended in pyridine (0.3 M) open to air. Thiocarbonyldiimidazole (1.1 equiv.) was added. The mixture was then stirred for 12 h at 35 °C.
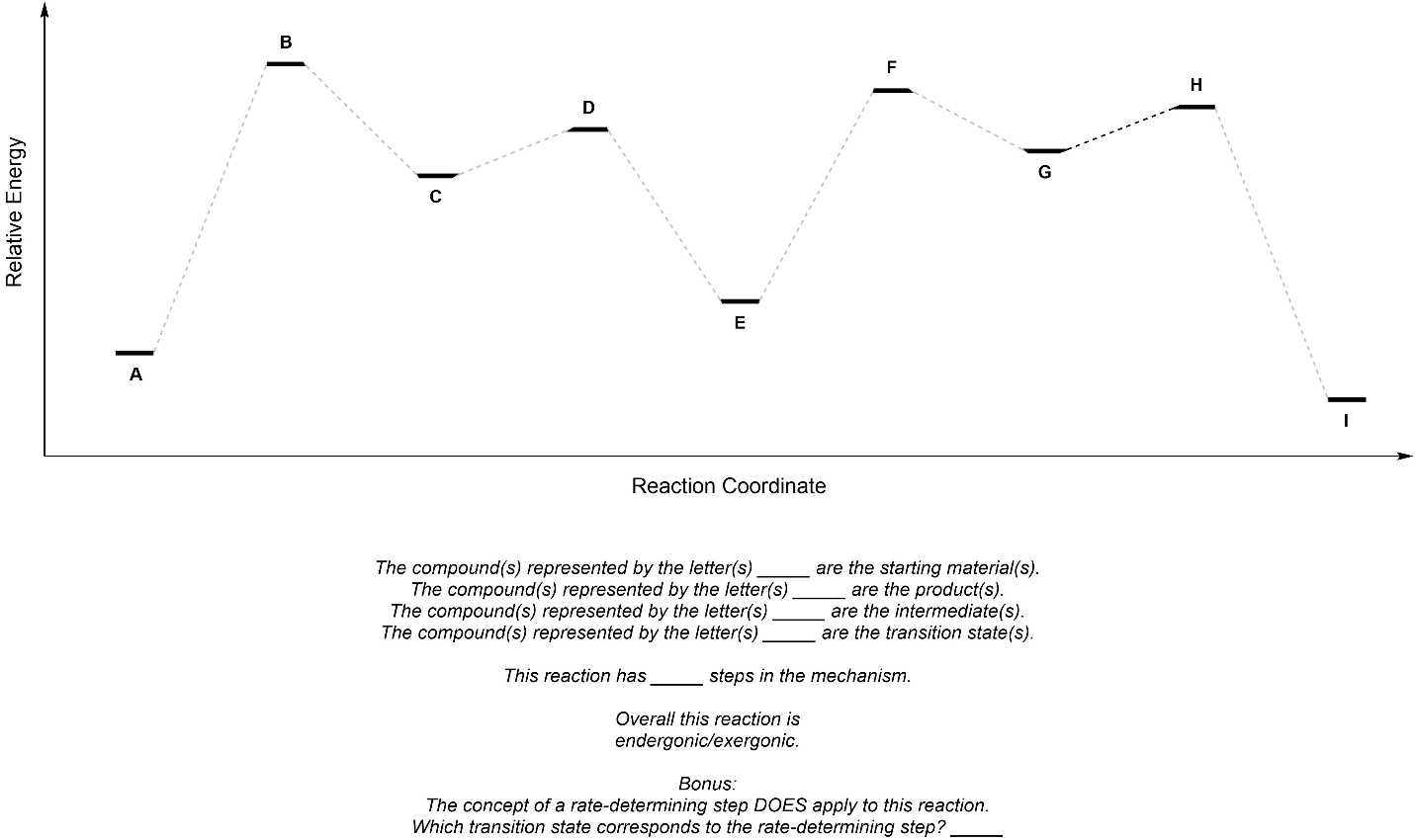
Q5.7: Fill in the blanks using the reaction coordinate below.