Chapter 4 Practice Problems – Answers
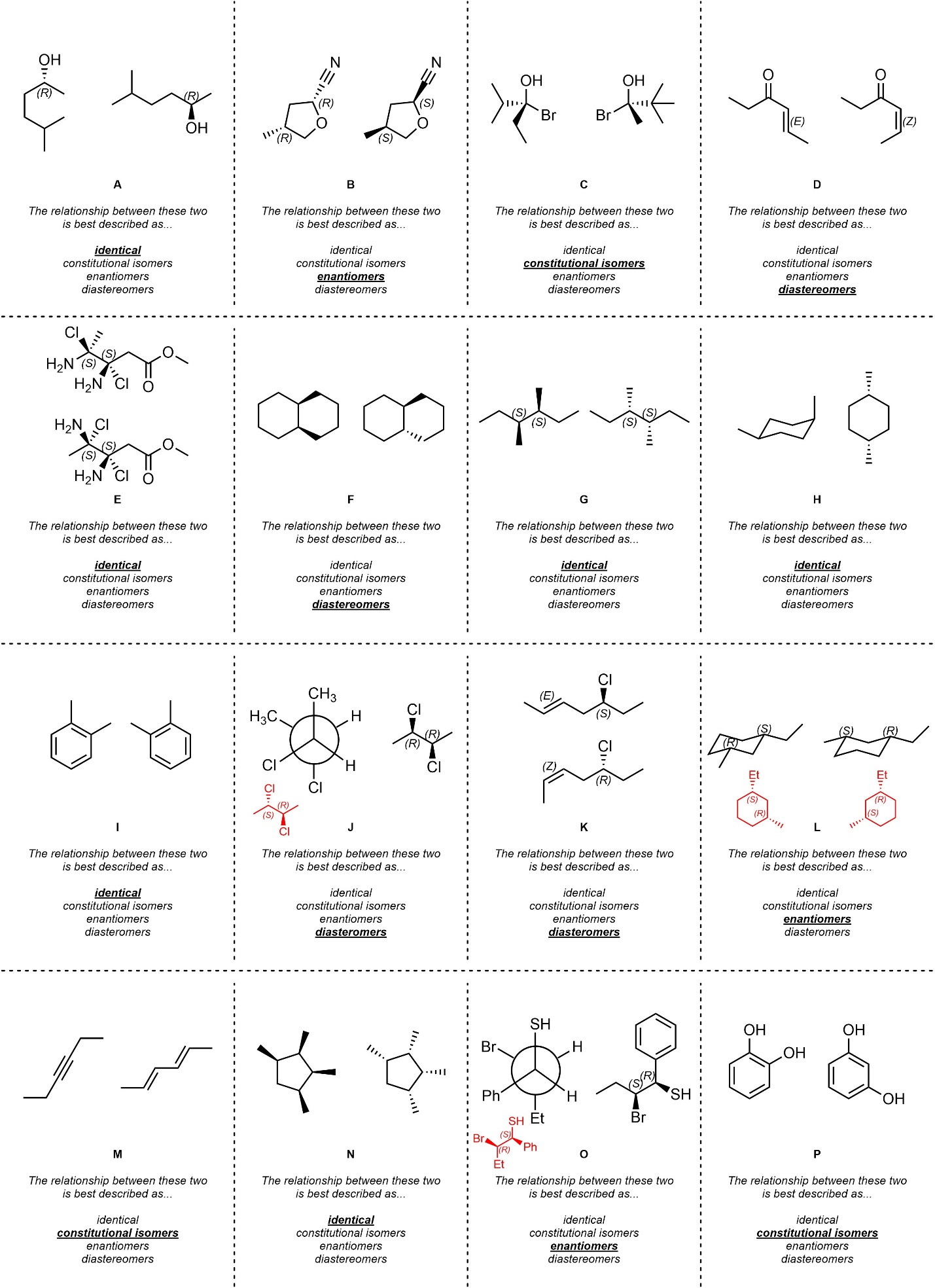
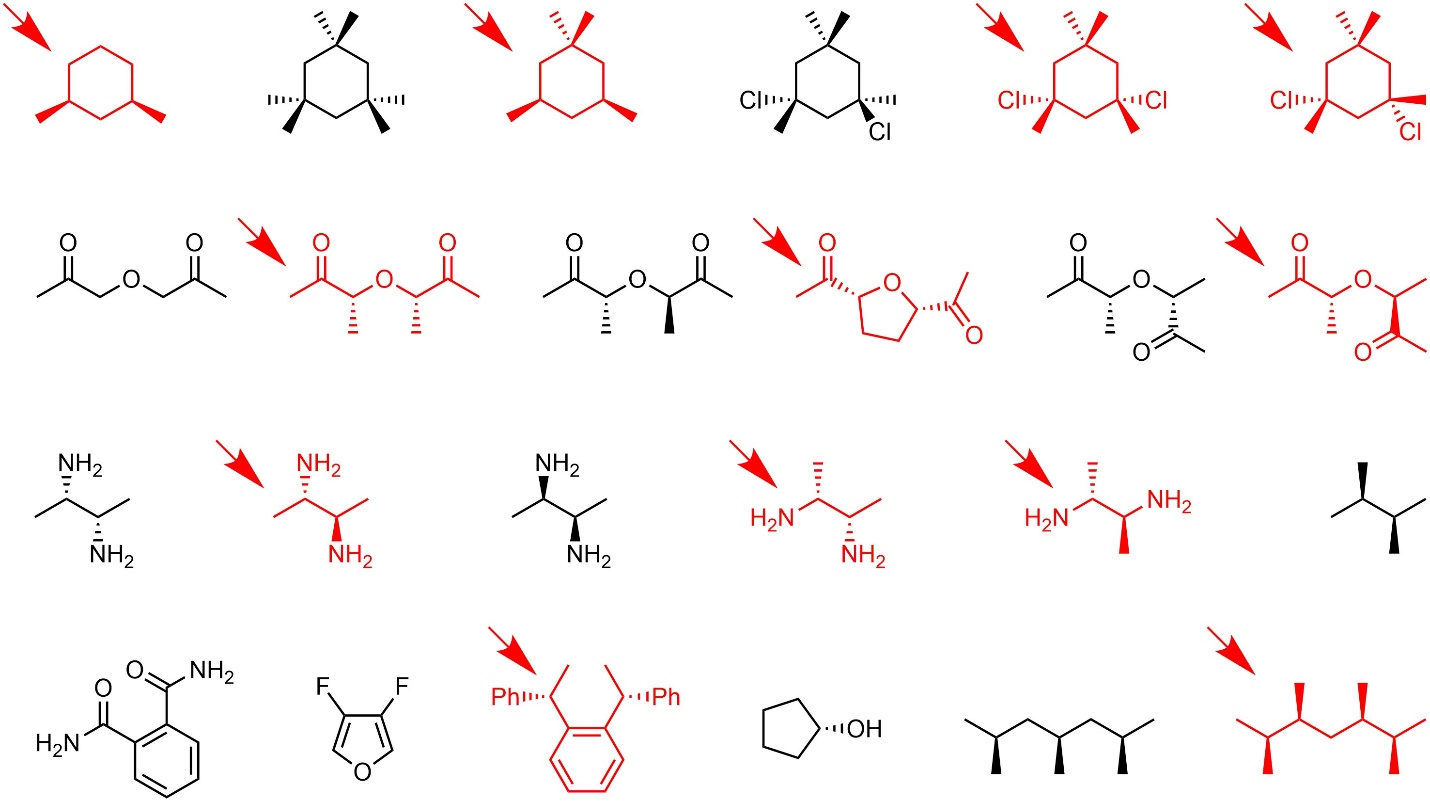
Q4.1: Class the relationship between each pair of molecule below as identical (including conformations of the same compound), constitutional isomers, enantiomers, or diastereomers.
Building molecular models will help.
Re-drawing chair and Newman projection structures
as line-angle structures will help but is not required.
The red structures are examples of re-drawn compounds.
In some cases the answer is most easily reached by
quickly assigning R/S and E/Z to all stereocentres.
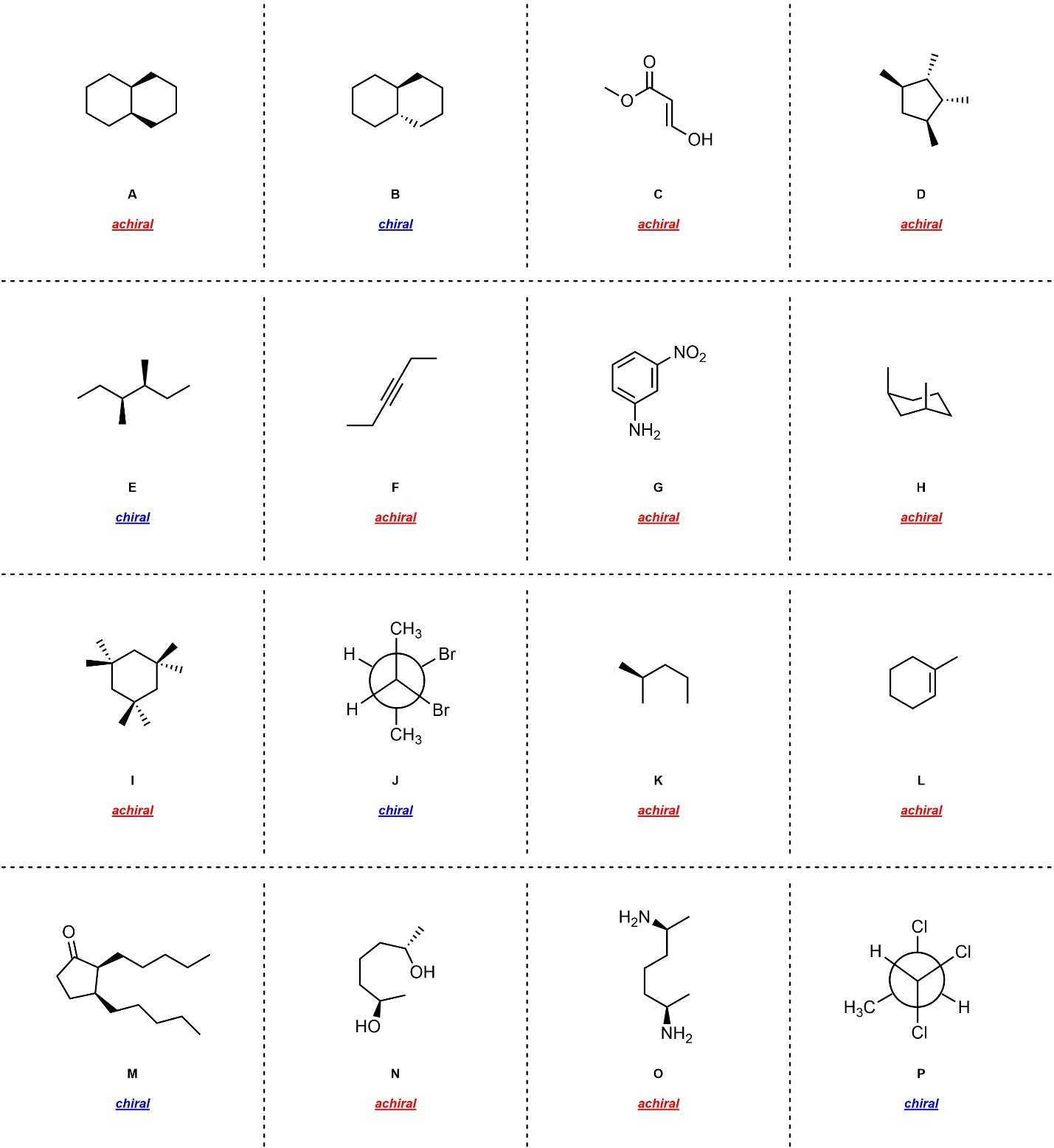
Q4.2: Assign each of the molecules below as chiral or achiral.
Building molecular models will help.
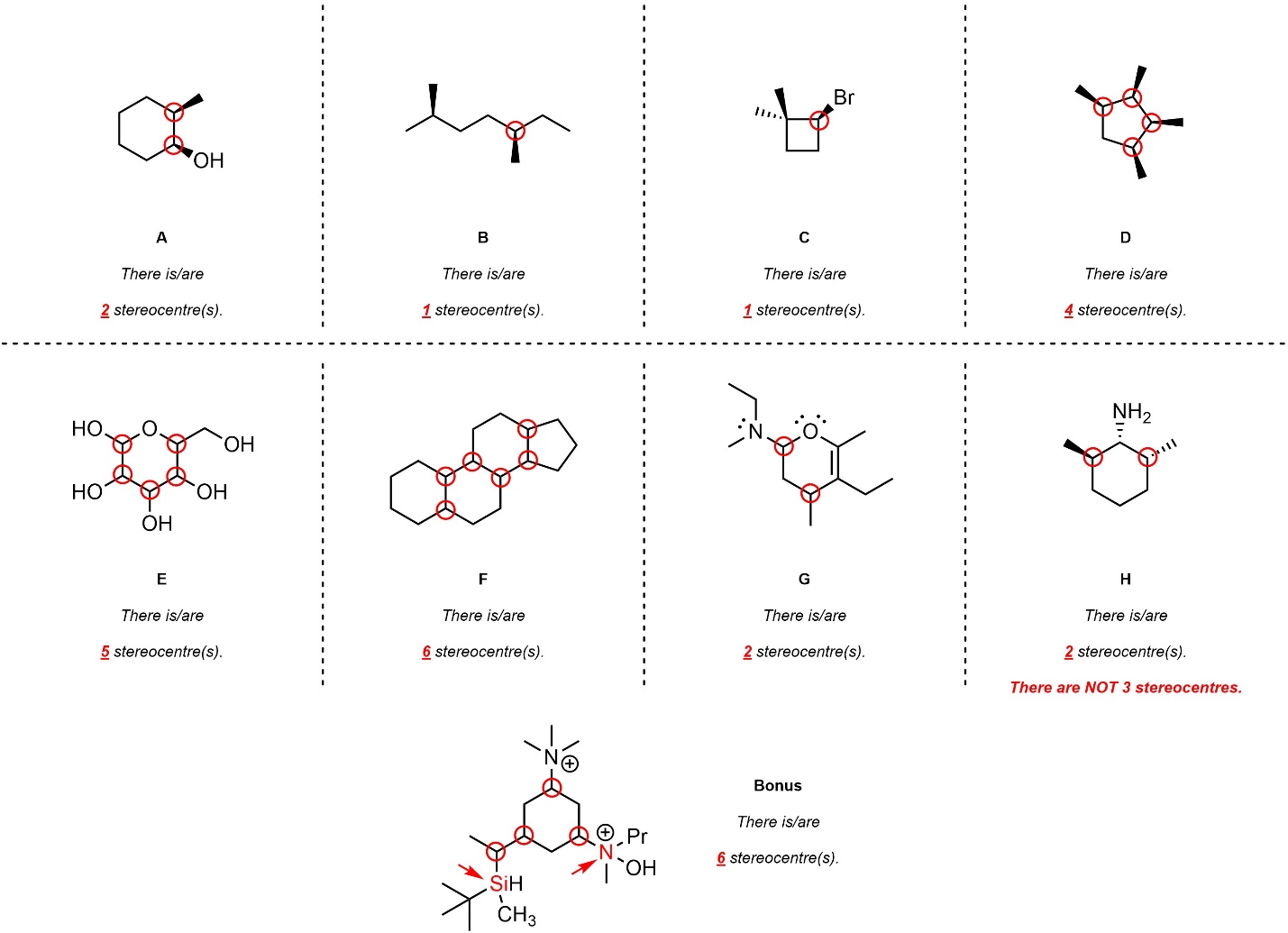
Q4.3: For each molecule below indicate (circle, draw an arrow to, etc.) all atoms that are stereogenic (stereocentres). Then write the total number of stereocentres for the molecule beneath it.
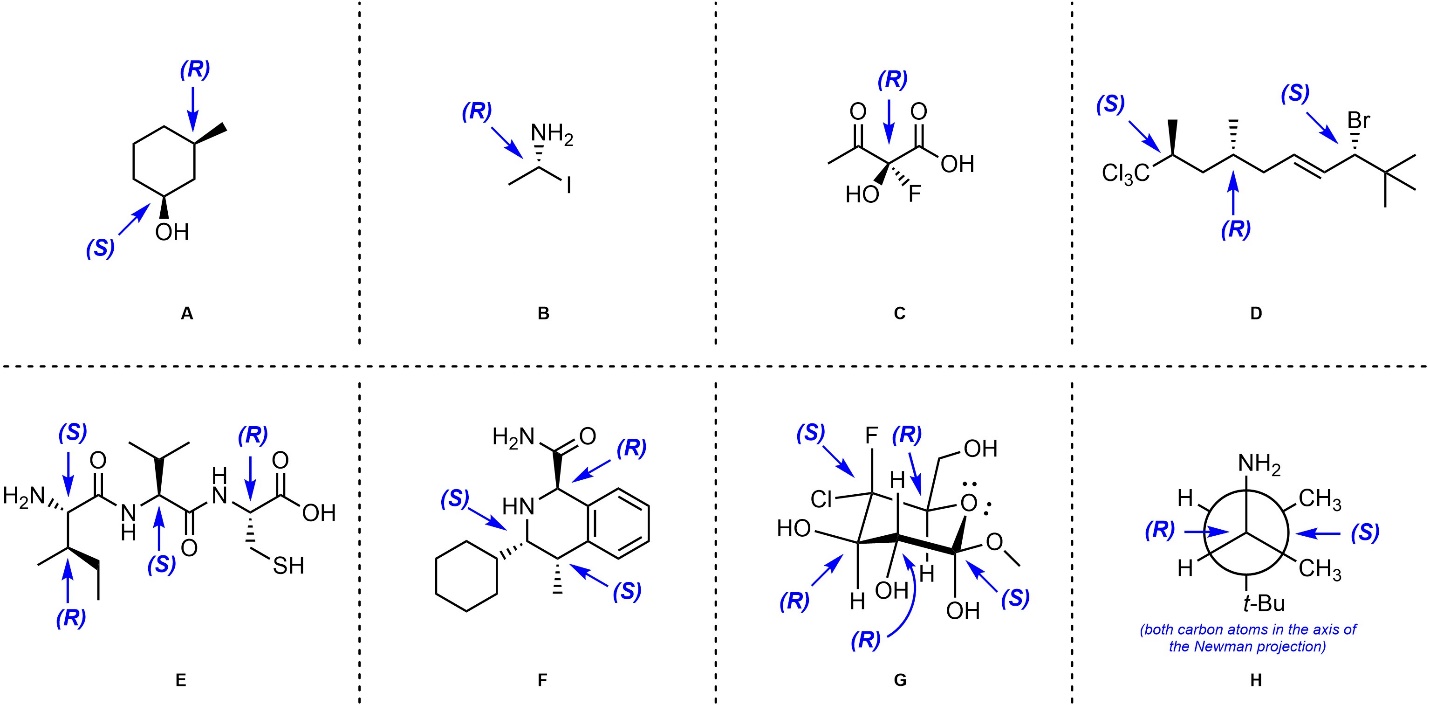
Q4.4: Assign absolute configurations (R/S) to each indicated stereocentre in the molecules below.
Having a Periodic Table handy will help.
Building a generic molecular model (Section 4.2.2.5) will help.
“Complex” examples (chairs, Newman projections) can be
converted into line-angle structures and then analyzed
OR
analyzed directly by visualizing them in 3D.
Q4.5: Indicate (circle, draw an arrow to, etc.) all molecules below that are meso.
Assigning absolute configurations (R/S) can help.
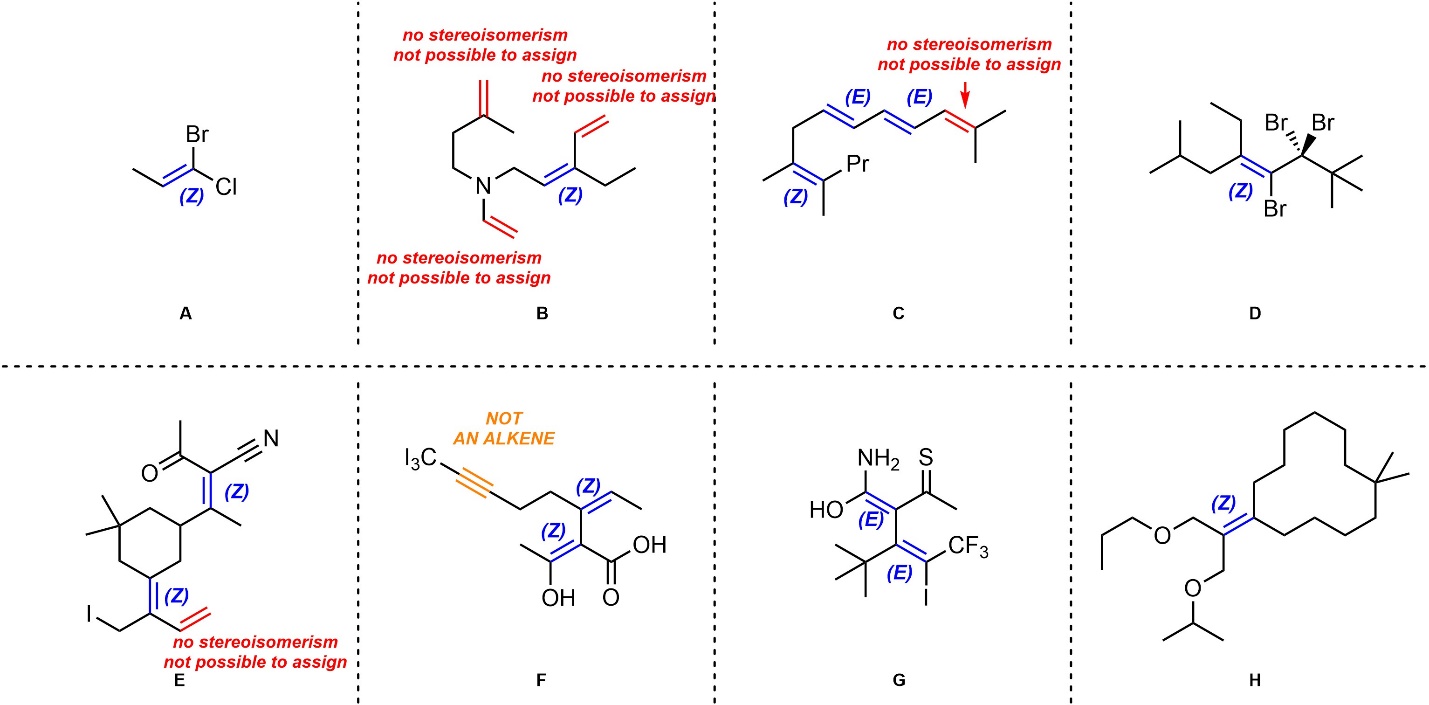
Q4.6: Assign absolute configurations (E/Z) to each alkene (if possible) in the molecules below. If it is not possible to assign an E/Z configuration to an alkene indicate this in some way beside it.
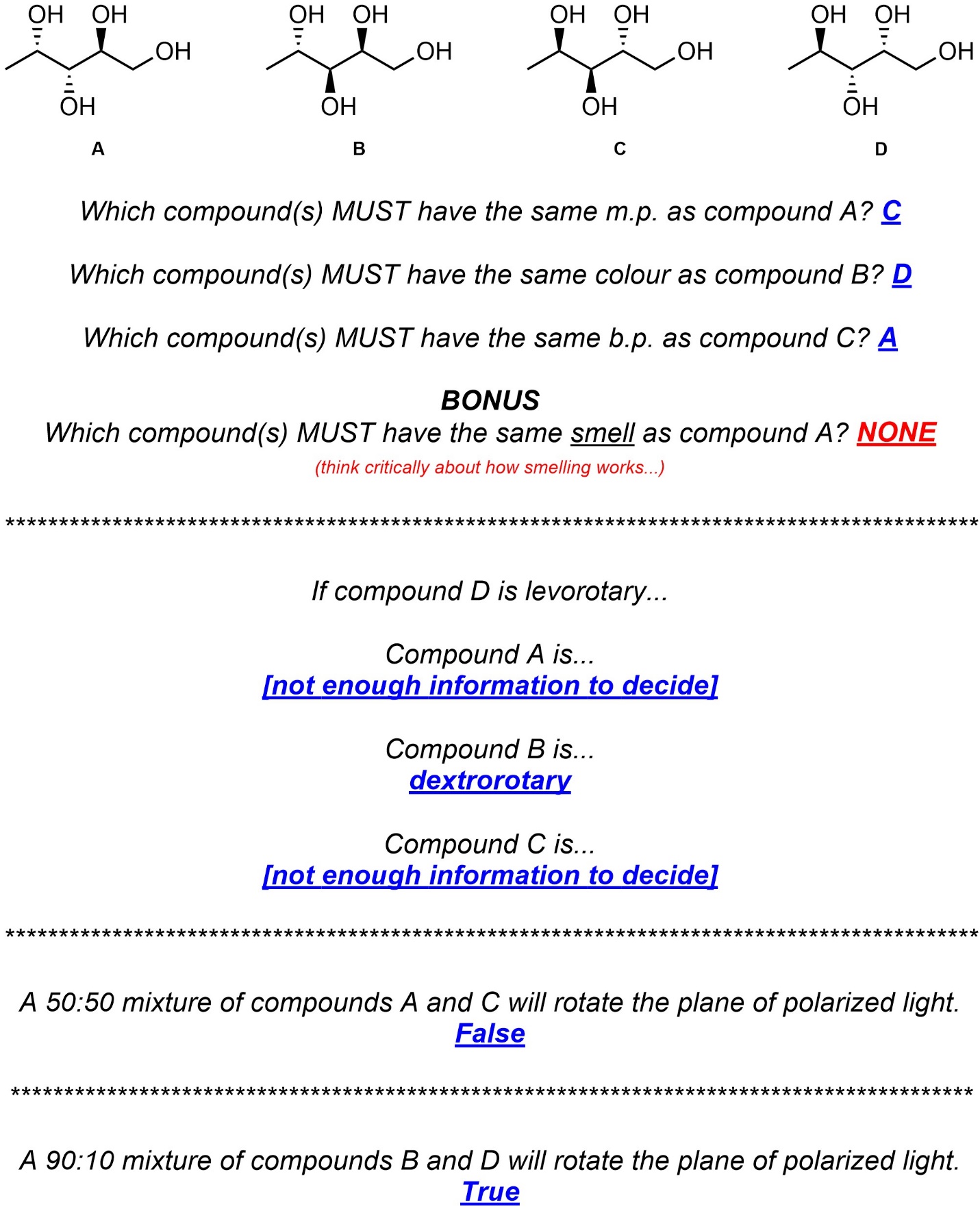
Q4.7: Answer the following questions about these four compounds by filling in the appropriate blanks and circling (highlighting, etc.) the most appropriate responses.